
Interpretation:
The products in the given cope rearrangements has to be predicted with stereochemistry.
Concept Introduction:
Cope-rearrangement:
It is a pericyclic reaction that involves the redistribution of six electrons through the formation of a cyclic transition state from which a
Example with mechanism of cope-arrangement:
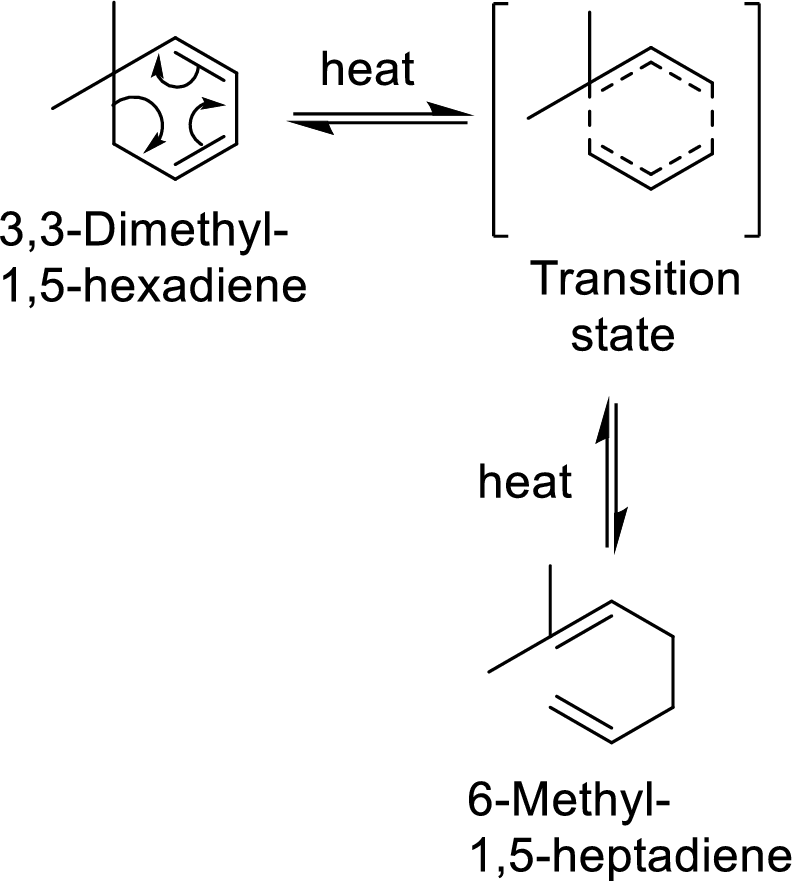
In this mechanism, two pi-bonds and one sigma bond of the reactant molecule has been rearranged and formed two new pi-bonds through a cyclic transition state.
Identification of cope-rearrangement in a
In the cope-rearrangement, the flow of electrons takes place between six bonds that are bonded as
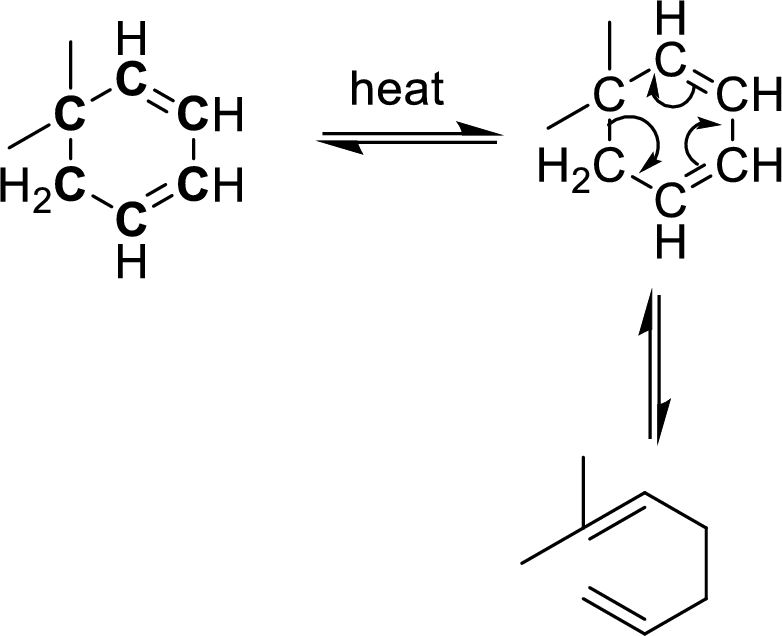
The carbon atoms that are involving in the cope-rearrangement are shown in bold.
Stereochemistry in a product formed:
- • In the product of a
chemical reaction , if a carbon atom has been attached with four different carbon atoms, then it is known as chiral carbon atom or stereocenter in the product. - • The bonds of the
functional groups because of which a new chiral carbon is supposed to form have to be represented in solid wedge bond and hashed-wedge bonds according to the particular enantiomer. - • Racemic mixture is the mixture of two enantiomers in equal proportions.
- • Enantiomers are non-superimposable mirror images.
- • Achiral product is the product in which there won’t be any chiral centre or stereocenter.
Trending nowThis is a popular solution!

Chapter 20 Solutions
Organic Chemistry, Loose-leaf Version
- I don't understand what to put for final step. Does that just mean termination? And would a radical form when I add bromine to ch2 between the rings?arrow_forwardNonearrow_forward11 1 Which one of the following compounds would show a proton NMR signal at the highest chemical shift? (7pts) cl @amitabh CI CI d) Cl CICIarrow_forward
- Nonearrow_forwardH2SO4 (cat.), H₂O 100 °C NH₂arrow_forwardX Draw the major products of the elimination reaction below. If elimination would not occur at a significant rate, check the box under the drawing area instead. ది www. Cl + OH Elimination will not occur at a significant rate. Click and drag to start drawing a structure.arrow_forward
- Nonearrow_forward1A H 2A Li Be Use the References to access important values if needed for this question. 8A 3A 4A 5A 6A 7A He B C N O F Ne Na Mg 3B 4B 5B 6B 7B 8B-1B 2B Al Si P 1B 2B Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe * Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha ****** Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Analyze the following reaction by looking at the electron configurations given below each box. Put a number and a symbol in each box to show the number and kind of the corresponding atom or ion. Use the smallest integers possible. cation anion + + Shell 1: 2 Shell 2: 8 Shell 3: 1 Shell 1 : 2 Shell 2 : 6 Shell 1 : 2 Shell 2: 8 Shell 1: 2 Shell 2: 8arrow_forwardNonearrow_forward
- IV. Show the detailed synthesis strategy for the following compounds. a. CH3CH2CH2CH2Br CH3CH2CCH2CH2CH3arrow_forwardDo the electrons on the OH participate in resonance with the ring through a p orbital? How many pi electrons are in the ring, 4 (from the two double bonds) or 6 (including the electrons on the O)?arrow_forwardPredict and draw the product of the following organic reaction:arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

