
Concept explainers
We now continue the use of
To make your own roadmap for Chapters 20–23, take a blank sheet of paper and write the following
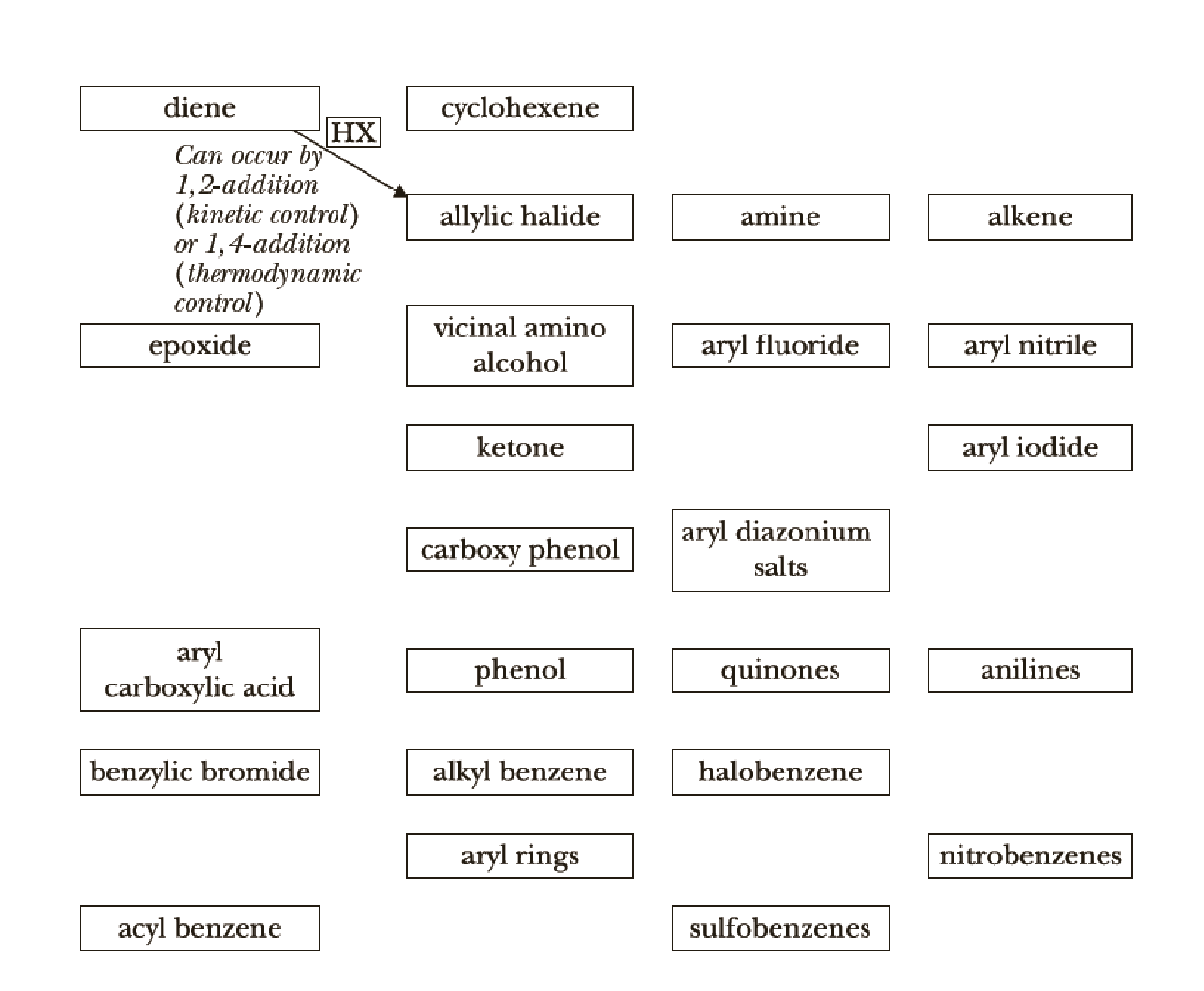
We now continue the use of organic chemistry reaction roadmaps. Because of the unique nature of the new reactions presented, we recommend that you make a new roadmap only for Chapters 20–23.
To make your own roadmap for Chapters 20–23, take a blank sheet of paper and write the following functional groups in the orientations shown. Fill the entire sheet of paper and leave plenty of room between functional groups. Most students find it helpful to use a poster-sized sheet of paper filled out in landscape orientation.
Interpretation:
A reaction roadmap has to be made for the reactions in the study Guide sections of chapters 20-23.
Concept Introduction:
Aromatic substitutions:
Aromatic substitution can be of two types, those are electrophilic and nucleophilic. Aromatic electrophilic substitution is an organic reaction in which an atom that is attached to an aromatic system is replaced by an electrophile. For example nitration, sulphonation, acylation, alkylation, halogenation to aromatic ring is aromatic electrophilic substitution reaction.
Aromatic nucleophilic substitution reactions are those reactions where nucleophile replaces a good leaving group.
Sigmatropic reaction:
A sigmatropic reaction is a pericyclic reaction where the net result is change of one sigma bond to another sigma bond in an uncatalysed intramolecular process.
Explanation of Solution
The reaction road maps for the reactions in the study Guide section of chapters (20-23) will help us to find out the reactions in easy way. The section number indicates the respective reaction segments involved in the study guide section.
Chapters 20 roadmap reaction legend,
Reaction 20.1:
Electrophilic addition to conjugate diene:
Conjugated diene undergoes both 1,2-addition and 1,4-addition reactions with electrophiles often giving mixtures of both kinds of products. The ratio of 1,2-addition to 1,4-addition is temperature dependent. 1,2-addition products are kinetically controlled product and 1,4 addition products are thermodynamically controlled product. When a conjugated diene reacts with
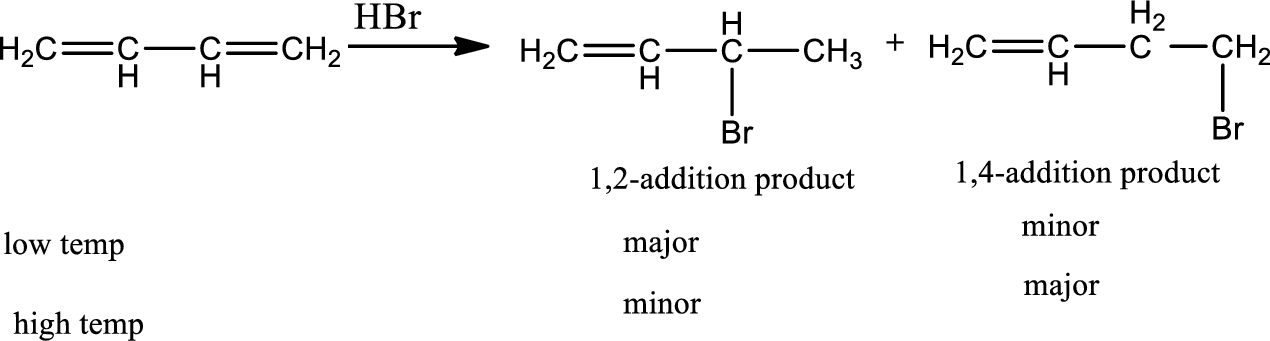
Reaction 20.2:
Diels-Alder reaction:
Conjugated dienes react with certain type of molecules having double or triple bonds, known as dienophile, to form two new sigma bond and a ring structure on the lose of pi bond in a reaction called Diels-Alder reaction. This reaction takes place in a concerted mechanism without intermediates and involves a redistribution of six pi electrons in a cyclic transition state where the pi bond breaking and new sigma bond making occurs simulteneously. The configuration of the diene and dienophile is preserved. Formation of the endo adduct is favoured. This reaction is facilitated by having electron withdrawing groups on dienophile and electron donating groups on diene. The diene must be on s-cis conformation to react. Diels-Alder reaction is an example of
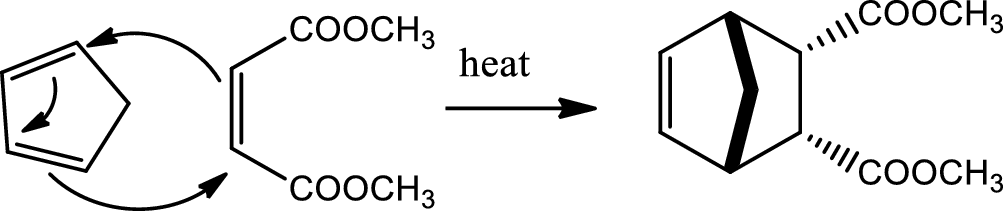
Reaction 20.3:
Claisen rearrangement:
The Claisen rearrangement is a powerful carbon-carbon bond forming chemical reaction. This rearrangement transforms an allyl phenyl ether to an ortho-substituted phenol. This rearrangement is an exothermic, concerted pericyclic reaction. The kinetics are first order and the whole transformation proceeds through a highly ordered cyclic transition state and it is intramolecular.
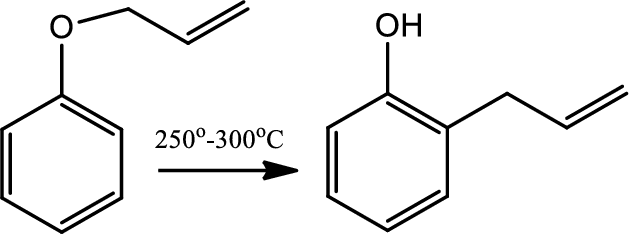
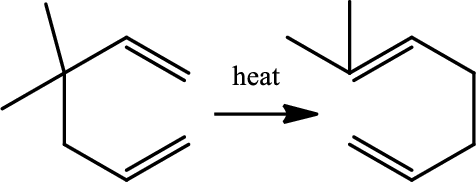
Reaction 20.4:
Cope rearrangement:
Cope rearrangement is the
Chapters 21 roadmap reaction legend,
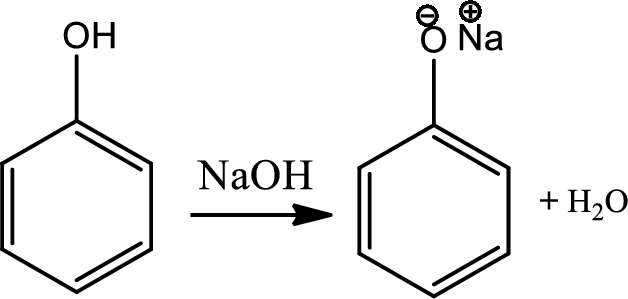
Reaction 21.1:
Acid base reaction of phenol:
Water soluble phenols react with the strong bases to give water soluble salts.
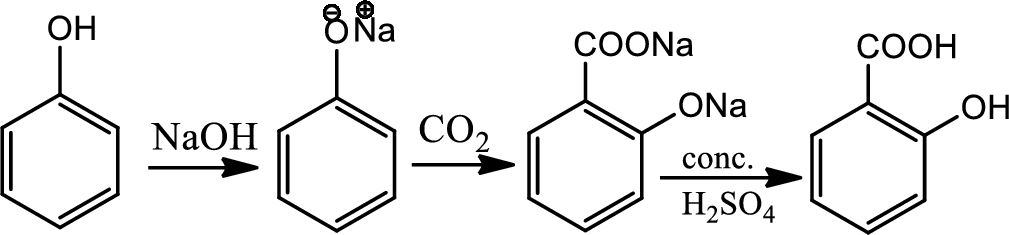
Reaction 21.2:
Kolbe synthesis:
Kolbe synthesis is a carboxylation chemical reaction that involves heating of sodim phenoxide with carbon dioxide and then treating the final product with sulfuric acid. This is basically nucleophilic addition of phenoxide to carbon dioxide that gives substituted cyclohexadienone which undergoes keto-enol tautomerism to regenerate aromatic ring. This gives maily ortho product which is known as salicylic acid.
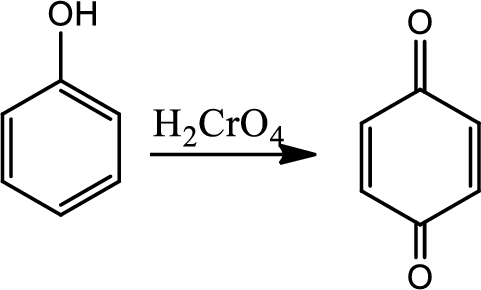
Reaction 21.3:
Oxidation of phenols to quinone:
Phenols can be easily oxidised to quinone. Oxidation of phenols by
Reaction 21.4:
Oxidation at benzylic position:
The benzylic carbon bonded hydrogen is weak and oxidation at benzylic position is to break the benzylic
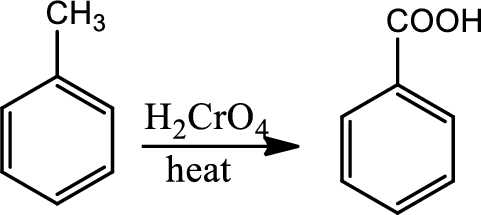
Reaction 21.5:
Halogenation at benzylic position:
Halogenation is regioselective for benzylic position and occurs by a radical chain mechanism. Bromination shows higher regioselectivity for a benzylic position than does chlorination. The reaction occurs via a radical chain mechanism which is initiated when the halohen molecule undergoes homolytic cleavage and gives two halide radical. One of the radical abstracts the benzylic hydrogen to create a resonance stabilised benzylic radical that reacts with another molecule of halogen to give the halogenated product and the new radical continues the chain reaction. NBS is used as the source of bromine.
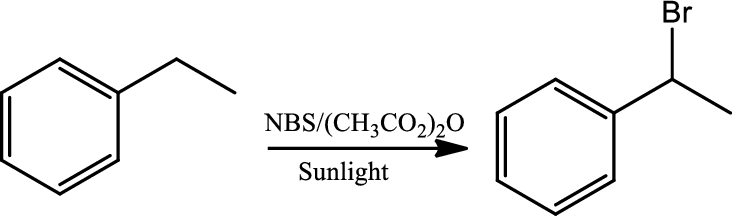
Reaction 21.6:
Hydrogenolysis of benzylic ethers:
Bezylic ethers can be cleaved to give an alcohol or toluene using catalytic hydrogenation of (
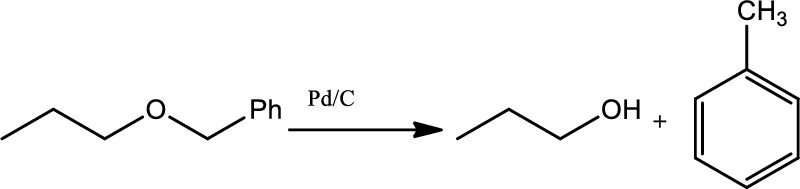
Chapters 22 roadmap reaction legend,
Reaction 22.1:
Halogenation:
In halogenation reaction, aromatic ring reacts with chlorine in the presence of lewis acid catalyst
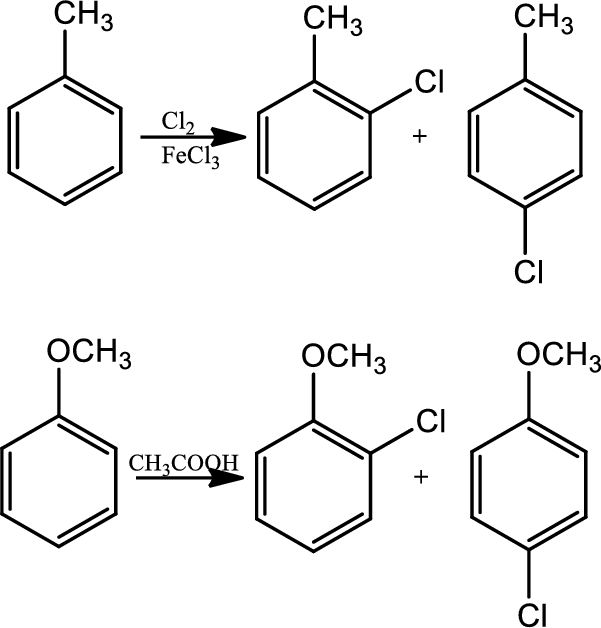
Reaction 22.2:
Nitration:
This is a type of electrophilic substitution on aromatic ring. The electrophile is the nitronium ion
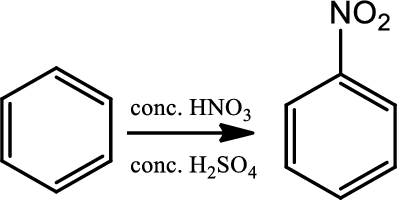
Reaction 22.3:
Sulfonation:
This is a type of electrophilic substitution on aromatic ring. The electrophile is either
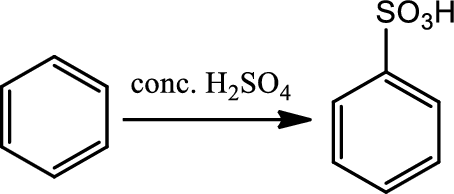
Reaction 22.4:
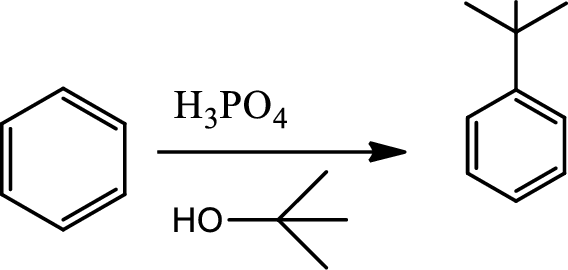
Fridel-craft’s alkylation:
This is a type of electrophilic substitution on aromatic ring. The electrophile is a carbocation formed as an ion pair interaction of haloalkane with lewis acid. Rearrangements from a less stable carbocation to more stable carbocation are common, the mechanism involves an initial reaction between the haloalkane and lewis acid
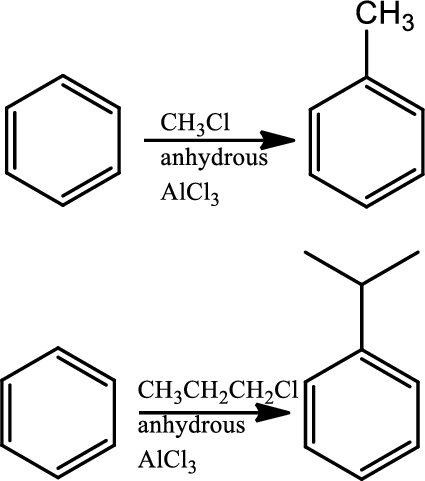
Reaction 22.5:
Fridel-craft’s acylation:
This is a type of electrophilic substitution on aromatic ring. The electrophile is an acylium cation formed as an ion pair by interaction of an acyl halide with a lewis acid. The mechanism involves an initial reaction between the acid chloride and lewis acid
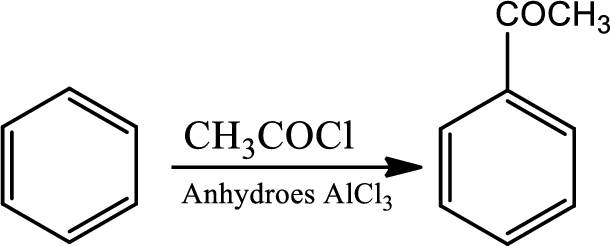
Reaction 22.6:
Alkylation using alkene:
This is a type of electrophilic substitution on aromatic ring. The electrophile is a carbocation fromed by the reaction between the alkene and acids.
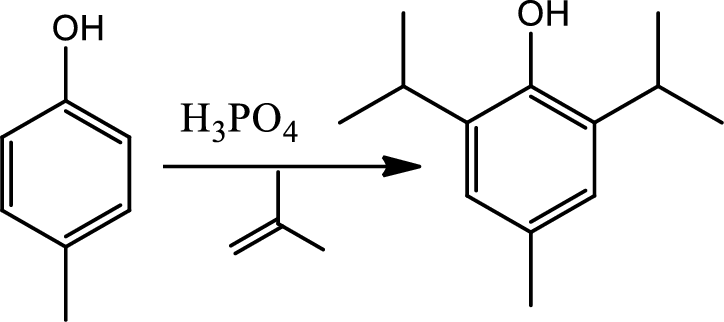
Reaction 22.7:
Alkylation using alcohols:
This is a type of electrophilic substitution on aromatic ring. The electrophile is a carbocation formed from an alcohol reacting with acid.
Chapters 23 roadmap reaction legend,
Reaction 23.1:
Alkylation of ammonia and amine:
Alkylation of amine is a type of organic reaction between an alkyl halide and ammonia or amine. This is nucleophilic aliphatic substitution and the reaction product is higher substitued amine. But the problem with the reaction is the overalkylation.
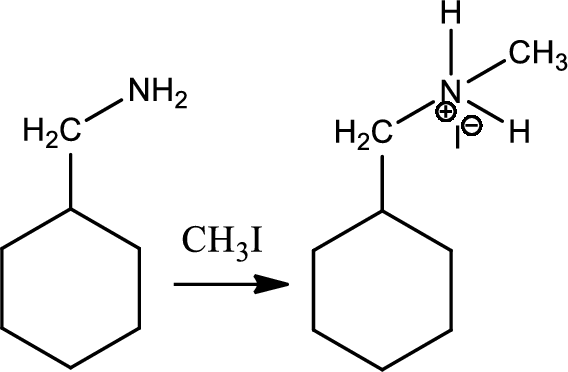
Reaction 23.2:
Alkylation of azide ion followed by reduction:
Azides are prepared by the treatment of a primary or secondary haloakane or an epoxide with
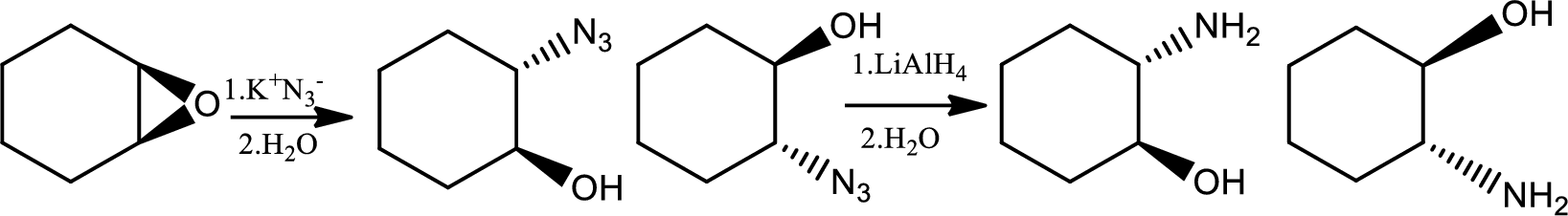
Reaction 23.3:
Nitrosation of tertiary aromatic amines:
This is an electrophilic aromatic substitution. The electrophile here is the nitrosyl cation which is very weak and participates in electrophilic aromatic substitution only with highly activated aromatic rings.
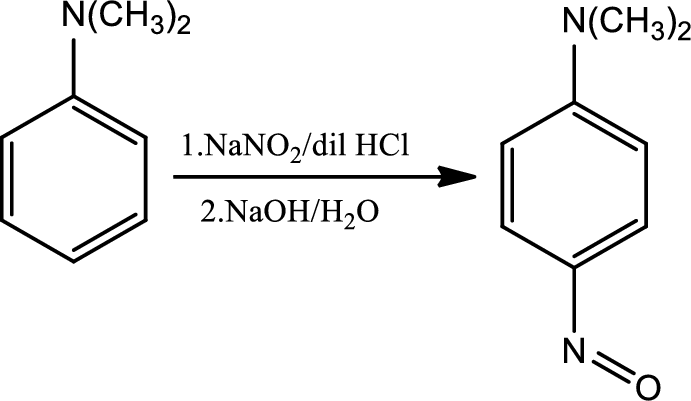
Reaction 23.4:
Formation of N-nitrosamine from secondary amines:
This is an electrophilic aromatic substitution. The electrophile here is the nitrosyl cation which reacts with secondary amine to give N-nitrsoamine.
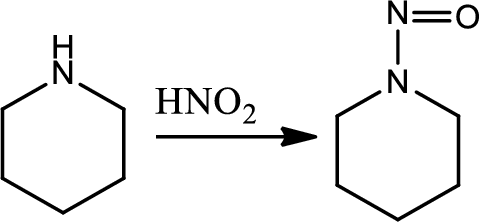
Reaction 23.5:
Diazotisation reaction:
Diazonium salta are organic compounds having
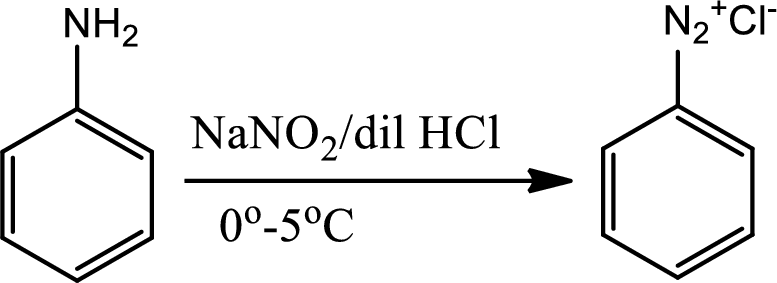
Reaction 23.6:
Conversion of primary aryl amine to phenol:
Formation of an arenediazonium salt followed by loss of nitrogen gives an aryl cation intermediate which then reacts with water to give a phenol.
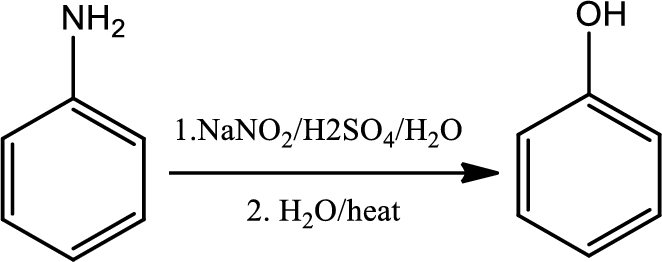
Reaction 23.7:
Schiemann reaction:
The Schiemann reaction is a chemical reaction in which a primary amine is transformed to an aryl fluoride via diazonium tetrafluoroborate intermediate.
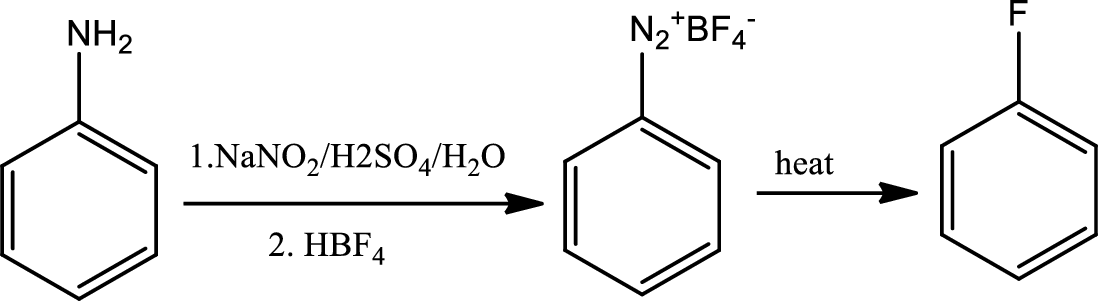
Reaction 23.8:
Sandmeyer reaction:
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which the unique transformation on benzene such as halogenation, cyanation, trifluoromethylation and hydroxylations can be done.
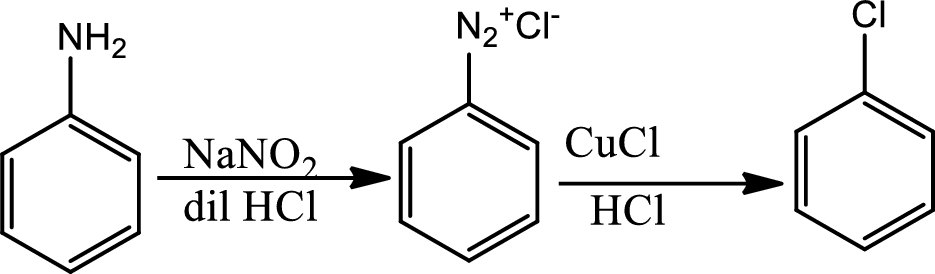
Reaction 23.9:
Reaction of arenediazonium salt with KI:
Reaction of arenediazonium salt with KI is the most convinient way of introducing iodide to benzene ring.
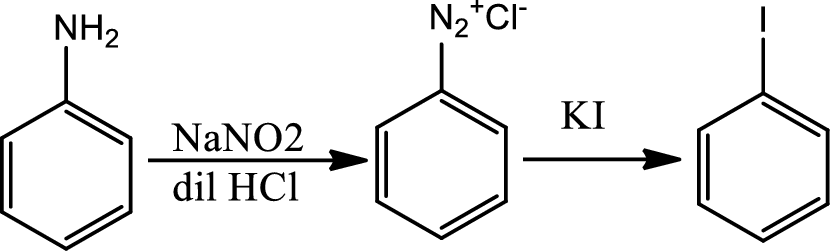
Reaction 23.10:
Reduction of arenediazonium salt with hypophosphorus acid:
By this pathway amine or nitro group can be removed from benzene ring.
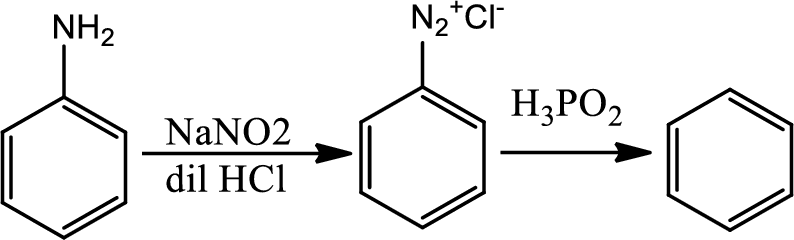
Reaction 23.11:
Hoffmann elimination:
Reaction of a quarternary ammonium halide with moist silver oxide to produce a quarternary ammonium hydroxide followed by heating to give an alkene is a reaction known as Hoffmann elimination. The mechanism involves the simultenous deprotonation of a beta hydrogen by base and los of the amino group in an anti geometry. In this process less substituted alkene is formed.
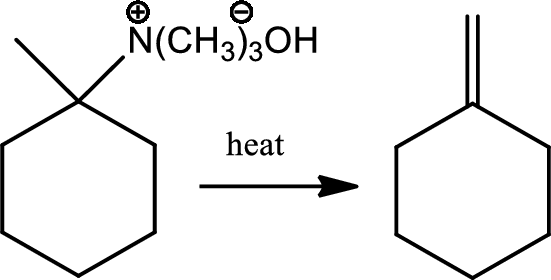
Reaction 23.12:
Cope elimination:
Treatment of the tertiary amine with hydrogen peroxide gives an amine oxide which on heating gives an alkene and an N,N-dialkylhydroxyamine in the reaction is known as Cope elimination. The elimination is syn stereoselective and involves a cyclic flow of six electrons in planar transition state.
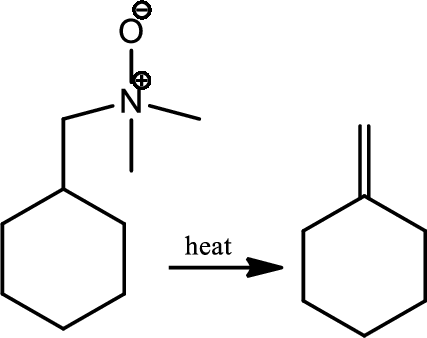
Reaction 23.12:
Reaction of cyclic beta amino alcohol with nitrous acid:
Treating a cyclic beta amino alcohol with nitrous acid leads to rearrangement and a ring expanded ketone. The mechanism involves an initial formation of diazonium ion followed by simulteneous loss of nitrogen and rearrangement by a 1,2-shift to give a resonance stabilised cation that loses a proton to give the product cyclic ketone.
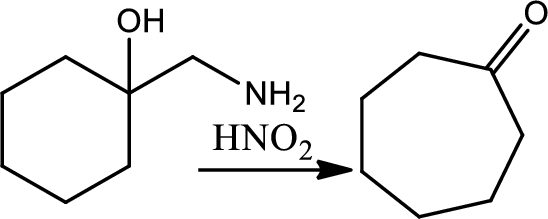
Thus, the reaction roadmap for the reactions in the study Guide section of chapters can be made as shown below for the chapters question 20-23,
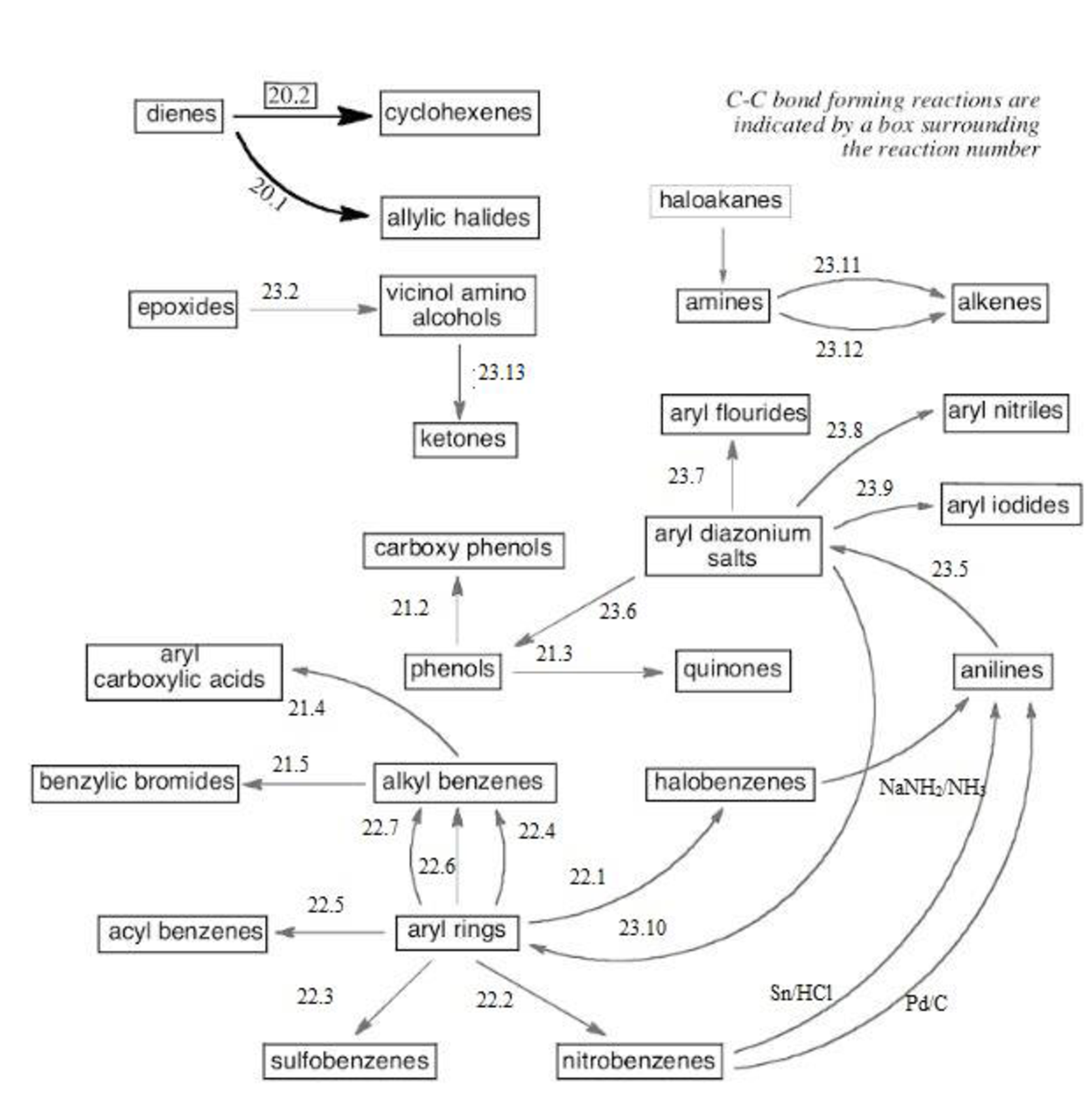
Figure-1
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry, Loose-leaf Version
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: 0.2 mol of KOH is added to 1.0 L of a 0.5 M HF solution. bases: Х other: ☐ acids: 0.10 mol of HI is added to 1.0 L of a solution that is 1.4M in both HF and NaF. bases: other: ☐ 0,0,... ด ? 18 Ararrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. acids: ☐ 1.8 mol of HCl is added to 1.0 L of a 1.0M NH3 bases: ☐ solution. other: ☐ 0.18 mol of HNO3 is added to 1.0 L of a solution that is 1.4M in both NH3 and NH₁Br. acids: bases: ☐ other: ☐ 0,0,... ? 000 18 Ar B 1arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NH3 (g) = N2 (g) +3H₂ —N2 (g) AGº = 34. kJ Now suppose a reaction vessel is filled with 4.19 atm of ammonia (NH3) and 9.94 atm of nitrogen (N2) at 378. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NH 3 tend to rise or fall? ☐ x10 fall Х Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH 3 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no atm 00. 18 Ar 무ㅎ ?arrow_forward
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 2.2 mol of NaOH is added to 1.0 L of a 1.4M HF solution. acids: П bases: Х other: ☐ ப acids: 0.51 mol of KOH is added to 1.0 L of a solution that is bases: 1.3M in both HF and NaF. other: ☐ 00. 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2O4 (g) 2NO2 (g) AG⁰ = 5.4 kJ Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2O4 tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. yes no 0.42 atm ☑ 5 0/5 ? مله Ararrow_forwardHomework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forward
- Identifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forwardHighlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward
- € + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forwardDraw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forwardEpoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
