
Concept explainers
(a)
Interpretation: The product formed when the
Concept introduction: The substitution reaction involves the replacement of one
Answer to Problem 20.41P
The product formed when the
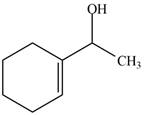
Figure 1
Explanation of Solution
The reaction that shows the formation of product when
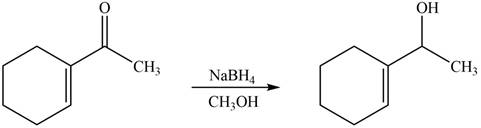
Figure 2
In the given reaction, sodium borohydride is used as a reducing agent. Sodium borohydride is used for the reduction of carbonyl compounds to alcohols.
The product formed when the
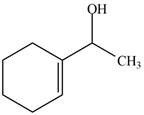
Figure 1
(b)
Interpretation: The product formed when the
Concept introduction: Addition of hydrogen takes place across a double bond of
Answer to Problem 20.41P
The product formed when the
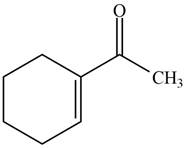
Figure 3
Explanation of Solution
The reaction that shows the formation of product when
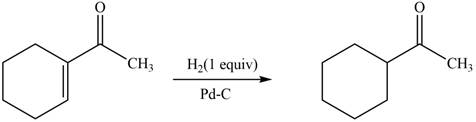
Figure 4
In the given reaction, hydrogenation of alkene takes place in the presence of palladium.
The product formed when the
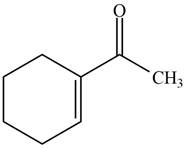
Figure 3
(c)
Interpretation: The product formed when the
Concept introduction: Addition of hydrogen takes place across a double bond of alkenes to form alkanes. This process is known as hydrogenation. It takes place in the presence of catalysts such as nickel, platinum or palladium.
Answer to Problem 20.41P
The product formed when the
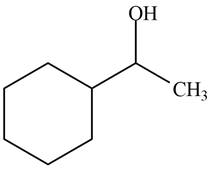
Figure 5
Explanation of Solution
The reaction that shows the formation of product when
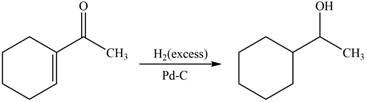
Figure 6
In the given reaction, excess hydrogen is used which will reduce both keto group and double bond. This results in the formation of a saturated compound.
The product formed when the

Figure 5
(d)
Interpretation: The product formed when the
Concept introduction: Methyllithium in the presence of water reduces carbonyl compounds into alcohol. Methyllithium is a reducing agent.
Answer to Problem 20.41P
The product formed when the
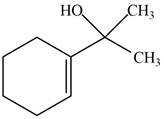
Figure 7
Explanation of Solution
The reaction that shows the formation of product when
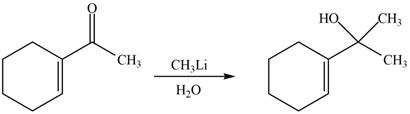
Figure 8
The product formed in the given reaction is
The product formed when the
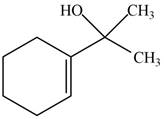
Figure 7
(e)
Interpretation: The product formed when the
Concept introduction: Grignard reagent is prepared by the reaction of alkyl or aryl bromide with magnesium metal in the presence of ether. The reaction of Grignard reagent with an
Answer to Problem 20.41P
The product formed when the
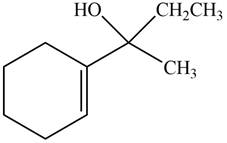
Figure 9
Explanation of Solution
The reaction that shows the formation of product when
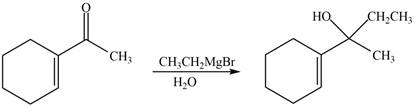
Figure 10
In the given reaction, Grignard reagent is used. This reagent attacks on the carbonyl compound to form alkoxides. In the next step, protonation with water takes place to give the final product.
The product formed when the
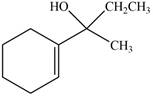
Figure 9
(f)
Interpretation: The product formed when the
Concept introduction: The steps involved in the reaction of ketone with organolithium reagent are as follows:
• The first step involves the reduction of carbonyl group into single bonds.
• The second step is the addition of R group from organometallic reagent and hydrogen to oxygen.
Answer to Problem 20.41P
The product formed when the
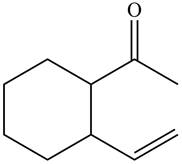
Figure 11
Explanation of Solution
The reaction that shows the formation of product when
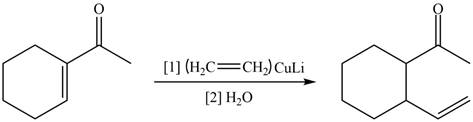
Figure 12
In the given reaction, organo copper reagent reacts with unsaturated carbonyl compound and forms carbon–carbon double bond with the beta carbon as shown in the above reaction.
The product formed when the

Figure 11
Want to see more full solutions like this?
Chapter 20 Solutions
ORGANIC CHEMISTRY
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





