
Draw the products formed when pentanal
a.
b.
c.
d.
e.
f.
g.
h.
i.
j.
k.
l. The [product in (a), then TBDMS-Cl, imidazole.
(a)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: The substitution reaction involves the replacement of one functional group by other functional group. In nucleophilic substitution an electron rich species attack the species that is deficient in electrons. The electrophile and the leaving group together form a substrate. The nucleophile attacks over the substrate and there occurs the removal of leaving group from the substrate.
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
![]()
Figure 1
Explanation of Solution
The reaction that shows the formation of product when pentanal reacts with
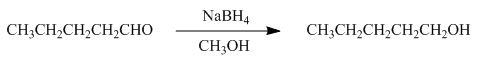
Figure 2
In the given reaction, sodium borohydride is used as a reducing agent. Sodium borohydride is used to for the reduction of carbonyl compounds to alcohols.
The product formed by the reaction of pentanal with
(b)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: The substitution reaction involves the replacement of one functional group by other functional group. In nucleophilic substitution an electron rich species attack the species that is deficient in electrons. The electrophile and the leaving group together form a substrate. The nucleophile attacks over the substrate and there occurs the removal of leaving group from the substrate.
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
![]()
Figure 1
Explanation of Solution
The reaction that shows the formation of product when pentanal reacts with
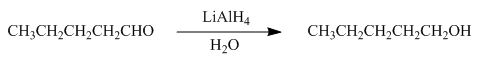
Figure 3
In the given reaction,
The product formed by the reaction of pentanal with
(c)
Interpretation: The product formed by the reaction of pentanal with the
Concept introduction: Addition of hydrogen takes place across a double bond of alkenes to form alkanes. This process is known as hydrogenation. It takes place in the presence of catalysts such as nickel, platinum or palladium.
Answer to Problem 20.38P
The product formed by the reaction of pentanal with the
![]()
Figure 1
Explanation of Solution
The reaction that shows the formation of product when
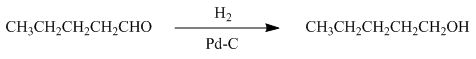
Figure 4
In the given reaction, hydrogenation of alkene takes place in the presence of palladium.
The product formed by the reaction of pentanal with the
(d)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction:
Answer to Problem 20.38P
No product is formed by the reaction of pentanal with
Explanation of Solution
The product formed by the reaction of pentanal with
![]()
Figure 5
No product is formed by the reaction of pentanal with
(e)
Interpretation: The product formed by the reaction of pentanal with the
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
The product formed by the reaction of pentanal with the
![]()
Figure 6
Explanation of Solution
The product formed by the reaction of pentanal with the
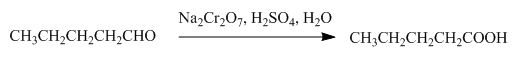
Figure 7
The product formed by the reaction of pentanal with the
(f)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction:
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
![]()
Figure 6
Explanation of Solution
The product formed by the reaction of pentanal with
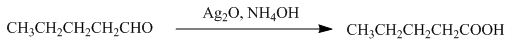
Figure 8
The product formed by the reaction of pentanal with
(g)
Interpretation: The product formed by the reaction of pentanal with the
Concept introduction: Grignard reagent is prepared by the reaction of alkyl or aryl bromide with magnesium metal in the presence of ether. The reaction of Grignard reagent with an aldehyde/ketone followed by hydrolysis yields an alcohol.
Answer to Problem 20.38P
The product formed by the reaction of pentanal with the
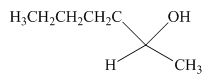
Figure 9
Explanation of Solution
The reaction that shows the formation of product when
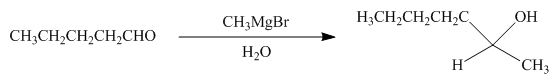
Figure 10
In the given reaction, Grignard reagent is used. This reagent attacks on the carbonyl compound to form alkoxides. In the next step, protonation with water takes place to give the final product.
The product formed by the reaction of pentanal with the
(h)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
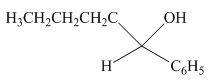
Figure 11
Explanation of Solution
The reaction that shows the formation of product when
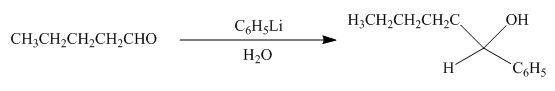
Figure 12
The product formed by the reaction of pentanal with
(i)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
No product formed by the reaction of pentanal with
Explanation of Solution
The product formed by the reaction of pentanal with
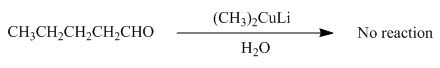
Figure 13
Aldehydes do not react with organo copper reagent because these reagents are less reactive and cannot attack to the electrophilic centre of the aldehyde group.
(j)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
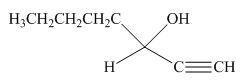
Figure 14
Explanation of Solution
The product formed by the reaction of pentanal with
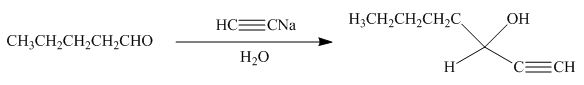
Figure 15
(k)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
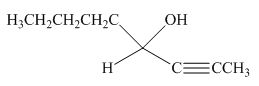
Figure 16
Explanation of Solution
The reaction that shows the formation of product when
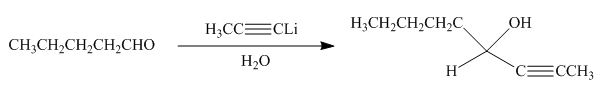
Figure 17
(l)
Interpretation: The product formed by the reaction of pentanal with
Concept introduction: Organometallic reagents like
Answer to Problem 20.38P
The product formed by the reaction of pentanal with
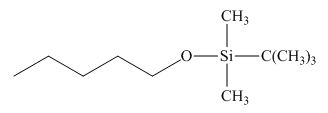
Figure 18
Explanation of Solution
The product formed by the reaction of pentanal with
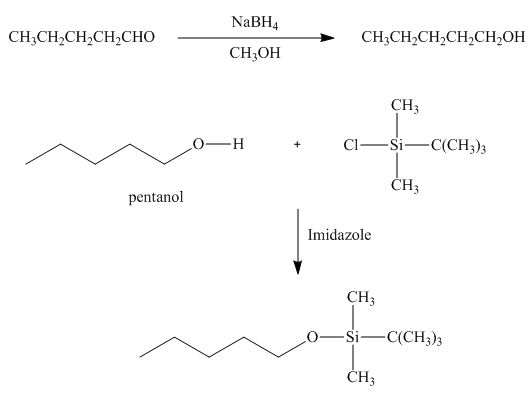
Figure 19
In the given reaction, sodium borohydride is used as a reducing agent. Sodium borohydride is used to for the reduction of carbonyl compounds to alcohols.
The product formed by the reaction of pentanal with the
Want to see more full solutions like this?
Chapter 20 Solutions
ORGANIC CHEMISTRY
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
- 5.arrow_forward6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward
- 5.arrow_forward9arrow_forwardalekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward
- 0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forwardx + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
