
Concept explainers
(a)
Interpretation:
The structure of an L- aldopentose needs to be drawn.
Concept introduction:
Sugar molecules can be named as D or L sugars according to their most oxidized carbon at the top of the Fisher projection. Aldopentose is a five-carbon
Answer to Problem 20.27P
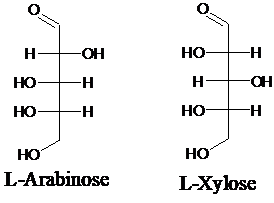
Explanation of Solution
Sugar molecules can be named as D or L sugars according to their most oxidized carbon at the top of the Fisher projection. The absolute configuration of a molecule can be explained using D and L configuration. In D- sugars, the OH group on the bottom chiral center points to the right while in L- sugars, the OH group on the bottom chiral center points to the left. Aldopentose is a five-carbon aldehyde sugar. Xylose, Arabinose and Ribose are few examples.
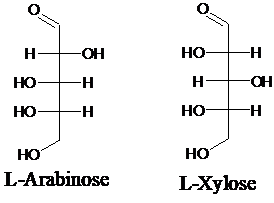
(b)
Interpretation:
The structure of a D- tetrose needs to be drawn.
Concept introduction:
Sugar molecules can name as D or L sugars according to their most oxidized carbon at the top of the Fisher projection. Aldotetrose is a four-carbon aldehyde sugar.
Answer to Problem 20.27P
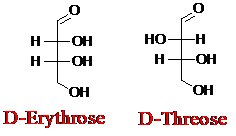
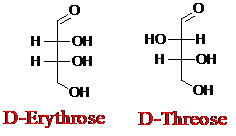
Explanation of Solution
Sugar molecules can be named as D or L sugars according to their most oxidized carbon at the top of the Fisher projection. The absolute configuration of a molecule can be explained using D and L configuration. In D- sugars, the OH group on the bottom chiral center points to the right while if L-sugars, the OH group on the bottom chiral center points to the left. Aldotetrose is a four-carbon aldehyde sugar.
(c)
Interpretation:
The structure of a five-carbon alditol needs to be drawn.
Concept introduction:
Alditols are polyols. Alditols can be produced by reducing an aldehyde or a
Answer to Problem 20.27P
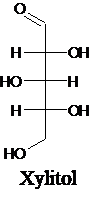
Explanation of Solution
Alditols are polyols. Alditols can be produced by reducing an aldehyde or a ketone group in a monosaccharide. In this reduction process, an aldehyde group or a ketone group converts into −CH2OH group. Xylitol is an example of an alditol.

For an example:
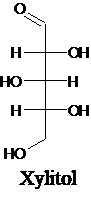
Want to see more full solutions like this?
Chapter 20 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
- Can anyone help me solve this step by step. Thank you in advaarrow_forwardPlease draw the mechanism for this Friedel-crafts acylation reaction using arrowsarrow_forwardDraw the Fischer projection of D-fructose. Click and drag to start drawing a structure. Skip Part Check AP 14 tv SC F1 F2 80 F3 a F4 ! 2 # 3 CF F5 75 Ax MacBook Air 894 $ 5olo % Λ 6 > W F6 K F7 &arrow_forward
- Consider this step in a radical reaction: Y What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ionization propagation initialization passivation none of the abovearrow_forward22.16 The following groups are ortho-para directors. (a) -C=CH₂ H (d) -Br (b) -NH2 (c) -OCHS Draw a contributing structure for the resonance-stabilized cation formed during elec- trophilic aromatic substitution that shows the role of each group in stabilizing the intermediate by further delocalizing its positive charge. 22.17 Predict the major product or products from treatment of each compound with Cl₁/FeCl₂- OH (b) NO2 CHO 22.18 How do you account for the fact that phenyl acetate is less reactive toward electro- philic aromatic substitution than anisole? Phenyl acetate Anisole CH (d)arrow_forwardShow how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forward
- Help me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forwardIs an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forward
- Help me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forwardV Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





