
Concept explainers
(a)
Interpretation:
The structure of
Concept introduction:
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
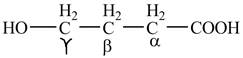
Explanation of Solution
The given compound contains four carbon atom hydrocarbon chain which is identified by butyric acid. It is also known as butanoic acid. Since its name ends with suffix
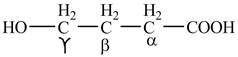
Figure 1
The structure of
(b)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
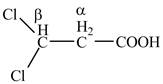
Explanation of Solution
The given compound contains three carbon atom hydrocarbon chain which is identified by propionic acid. It is also known as propanoic acid. Since its name ends with suffix
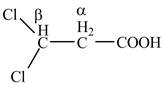
Figure 2
The structure of
(c)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
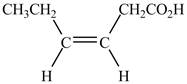
Explanation of Solution
The given compound contains six carbon atom hydrocarbon chain which is identified by hexenoic acid. It is an
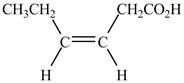
Figure 3
The structure of
(d)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
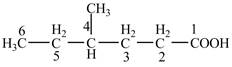
Explanation of Solution
The given compound contains six carbon atom hydrocarbon chain which is identified by hexanoic acid. Since its name ends with suffix
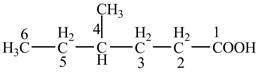
Figure 4
The structure of
(e)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
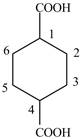
Explanation of Solution
The given compound contains six carbon atom hydrocarbon cyclic chain which is identified by cyclohexane. Since its name ends with dicarboxylic acid which means it contain two carboxyl groups and their position is
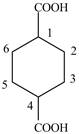
Figure 5
The structure of
(f)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
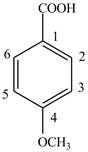
Explanation of Solution
The given compound contains six carbon atom
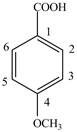
Figure 6
The structure of
(g)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of
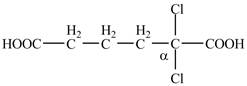
Explanation of Solution
The given compound contains six carbon atom hydrocarbon chain which is identified by adipic acid. Adipic acid is also known as
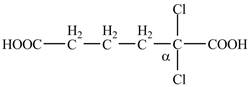
Figure 7
The structure of
(h)
Interpretation:
The structure of oxalic acid is to be drawn.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.1P
The structure of oxalic acid is shown below.
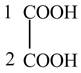
Explanation of Solution
The given compound contains two carbon atom hydrocarbon chain which is identified by oxalic acid. It is also known as
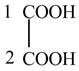
Figure 8
The structure of oxalic acid is shown in Figure 8.
Want to see more full solutions like this?
Chapter 20 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- a. Determine whether each of the Followery Molecules is in the R- On the y- Configuration 1-01"/ 1-6-4 Br 4 I el Br b. Draw The Fisher projection For all the Meso compounds that can exist FOR The Following molenlearrow_forward1- Refer to the monosaccharides below to answer each of the following question(s): CH₂OH CHO CH₂OH CH₂OH 0 H- OH 0 0 HO- H H- -OH HO H HO H H OH HO- H CH₂OH H. OH HO H HO- H CH₂OH CH₂OH CH3 a. Sorbose b. Rhamnose c. Erythrulose d. Xylulose Classify each sugar by type; for example, glucose is an aldohexose. a. Xylulose is .. b. Erythrulose is . c. Sorbose is .. d. Rhamnose is .. 2- Consider the reaction below to answer the following question(s). CHO H OH CH₂OH CH₂OH HO- H HO HO + H. -OH HO OH HO. H OH OH H -OH H OH CH₂OH Q Z a. Refer to Exhibit 25-11. Place a triangle around the anomeric carbon in compound Q. Compound Z is: b. 1. the D-anomer. 2. the a-anomer. 3. the ẞ-anomer. 4. the L-anomer. c. Which anomer is the LEAST stable? d. Q and Z are cyclic examples of: a. acetals b. hemiacetals c. alditols d. hemialditolsarrow_forwardi need help identifying the four carbon oxygen bonds in the following:arrow_forward
- Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule HO H3N + The solution is... X O acidic OH O basic H3N-CH-C-O O neutral ○ (unknown) O acidic ○ basic CH2 CH 3-S-CH2 O neutral ○ (unknown) H3N O OH O acidic O basic Oneutral O (unknown) 0 H3N-CH-C-O CH3 CH CH3 O acidic O basic O neutral ○ (unknown) ? olo Ar BHarrow_forwardno Ai walkthroughs need other product (product in picture is wrong dont submit the same thing)arrow_forwardHow to solve this!arrow_forward
- I have a 2 mil plastic film that degrades in 22 days at 88C and 153 days at 61C what is the predicted theoretical degradation at 47C?arrow_forwardno ai walkthrougharrow_forwardI have a 2 mil plastic film that degrades after 22 days at 88C and at 61C takes 153 days. What is the failure at 47C in days.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
