
Concept explainers
A plasmid that is both ampicillin and tetracycline resistant is cleaved with Pstl, which cleaves within the ampicillin resistance gene. The cut plasmid is ligated with Pstl-digested Drosophila DNA to prepare a genomic library, and the mixture is used to transform E. coli K12.
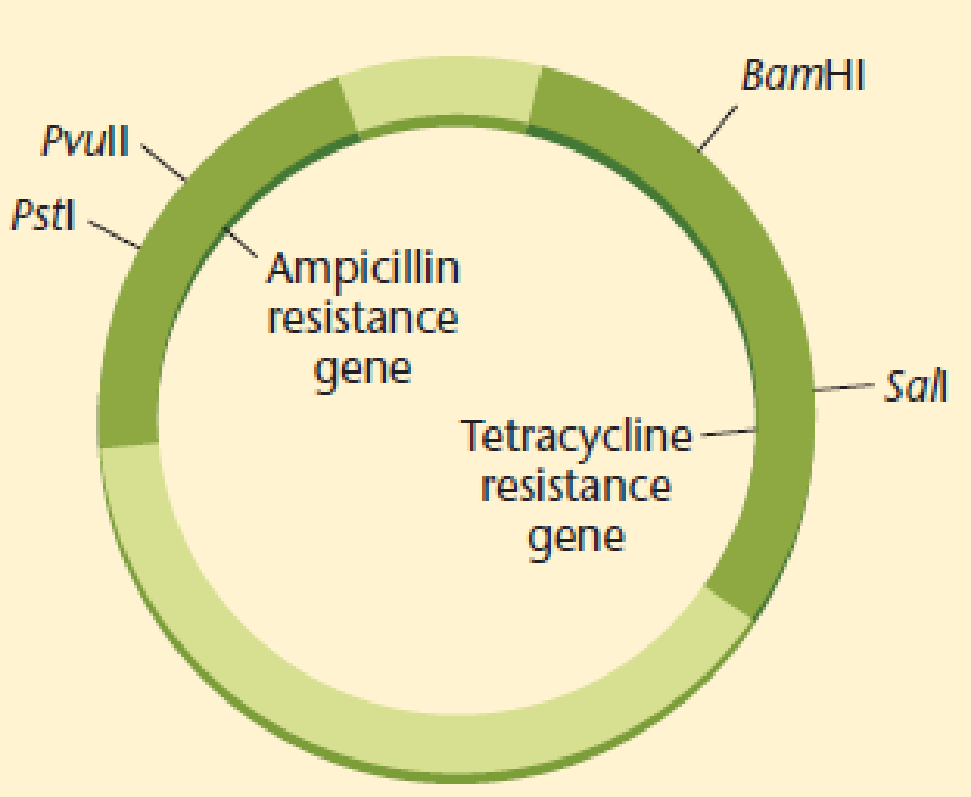
- (a) Which antibiotic should be added to the medium to select cells that have incorporated a plasmid?
- (b) If recombinant cells were plated on medium containing ampicillin or tetracycline and medium with both antibiotics, on which plates would you expect to see growth of bacteria containing plasmids with Drosophila DNA inserts?
- (c) How can you explain the presence of colonies that are resistant to both antibiotics?
(a)
To determine: The antibiotic that an individual should add to the medium to select cells that contain the recombinant plasmid.
Introduction: Plasmid refers to an circular, extrachromosomal DNA (deoxyribonucleic acid) molecule that replicates chromosome independently.
Explanation of Solution
The gene that confers resistance to tetracycline is intact in the recombinant plasmid. An individual would add tetracycline to the medium to select the recombinant plasmd in the cells. In the medium, the bacteria that have been transformed with recombinant plasmid will be resistant to tetracycline.
Thus, an individual would use tetracycline to select cells that contain the recombinant plasmid.
(b)
To determine: The plates on which an individual might see the growth of bacteria having plasmids with Drosophila DNA inserts.
Introduction: Genetically modified bacterial plasmids were the first developed vectors, used for cloning purpose.
Explanation of Solution
Cloning is a important process in which bacterial cells containing recombinant DNA can be readily identified. This process is also accomplished is through the use of selectable marker genes. Antibiotic resistant genes provide very effective selectable marker genes. Therefore, the colonies that grow on tetracycline medium but do not grow on ampicillin medium would contain the Drosophila DNA insert.
Thus, colonies that only grow in tetracycline medium probably contain the Drosophila DNA insert.
(c)
To determine: The reason that some colonies can grow on tetracycline medium as well as ampicillin medium.
Introduction: Plasmids have an origin of replication (ori) site, which makes it possible to produce several hundred copies of a plasmid in a single host cell.
Explanation of Solution
When performing cloning process, it is not necessary for incorporation of all DNA plasmids need to be cloned. A plasmid cut with a restriction enzyme generating sticky ends can self-ligate if cut ends of the plasmid rejoin. Also, if cleavage with PstI was incomplete, then no change in biological characteristics of the uncut plasmid would be expected.
Thus, self-ligation of plasmid and incomplete cleavage with PstI are responsible for the growth of colonies that are resistant to both antibiotics.
Want to see more full solutions like this?
Chapter 20 Solutions
Concepts of Genetics (12th Edition)
- answer questions 1-10arrow_forwardAnswer Question 1-9arrow_forwardEx: Mr. Mandarich wanted to see if the color of light shined on a planthad an effect on the number of leaves it had. He gathered a group ofthe same species of plants, gave them the same amount of water, anddid the test for the same amount of time. Only the color of light waschanged. IV:DV:Constants:Control Gr:arrow_forward
- ethical considerations in medical imagingarrow_forwardPlease correct answer and don't used hand raiting and don't used Ai solutionarrow_forward2. In one of the reactions of the citric acid cycle, malate is oxidized to oxaloacetate. When this reaction is considered in isolation, a small amount of malate remains and is not oxidized. The best term to explain this is a. enthalpy b. entropy c. equilibrium d. free energy e. loss of energyarrow_forward
- 18. The citric acid cycle takes place in a. the chloroplasts b. the cytosol c. the inner mitochondrial membrane d. between the two mitochondrial membranes e. the mitochondrial matrix 40 WILarrow_forward8. Most reactions of anaerobic respiration are similar to a. aerobic respiration b. photosynthesis c. lactic acid fermentation d. alcoholic fermentation e. both c and darrow_forward12. Which of the following molecules can absorb light? a. Pigments b. Chlorophyll c. Rhodopsin d. Carotenoids e. All of the abovearrow_forward
- Which of the following proteins or protein complexes is directly required for the targeting of mitochondrial inner membrane multipass proteins, such as metabolite transporters, whose signal sequence is normally not cleaved after import? OA. TIM22 OB. TIM23 C. OXA OD. Mia40 OE SAMarrow_forwardQUESTION 9 An animal cell has been wounded and has a small rupture in its plasma membrane. Which of the following is more likely to happen next? OA. The cell rapidly cleaves by cytokinesis. OB. The rate of receptor-mediati endocytosis is increased. OC. The rate of exocytosis is increased. OD. The rate of pinocytosis is increased.arrow_forwardFor the a subunit of a trimeric G protein, A. a G-protein-coupled receptor GPCR) acts as a guanine nucleotide exchange factor (GEF), whereas a regulator of G protein signaling (RGS) can act as a GTPase-activating protein (GAP). B. a GPCR acts as a GAP, whereas an RGS can act as a GEF. C. both a GPCR and an RGS can act as a GEF. O D. both a GPCR and an RGS can act as a GAP OE. None of the above.arrow_forward
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage LearningCase Studies In Health Information ManagementBiologyISBN:9781337676908Author:SCHNERINGPublisher:Cengage
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage LearningCase Studies In Health Information ManagementBiologyISBN:9781337676908Author:SCHNERINGPublisher:Cengage





