
CHEMISTRY:PRIN.+REACTIONS-OWLV2 ACCESS
8th Edition
ISBN: 9781305079298
Author: Masterton
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 2, Problem 59QAP
Interpretation Introduction
Interpretation:
To give all the formulas of compounds containing no other ions than K+, Ca2+, Cl-, S2-.
Concept introduction:
There are two types of ions:
Cations: These are positively charged ions formed by losing electrons.
Anions: These are negatively charged ions formed by gaining electrons.
Cations and anions combine to form ionic compounds.
Steps to write chemical formula from ions:
Let’s say we have two ions A+3 and B-2.
- Identify the cations and anions.
- Write both cation and anion together (placing cation first) as:
Cations are positively charged, and anions are negatively charged.
Here
A+3 = cation
B-2 = anion
- Put a value in the subscript of anion which is equal in magnitude with charge of cation and in the subscript of cation, put the value equals to magnitude of charge of anion as:
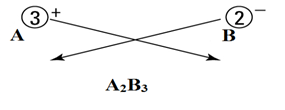
- If the subscript of anions and cations are multiple of a common number, then make it the smallest whole number ratio.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
7)
8)
FCI II
-C-C-C=C-C
||
Br Br
||
-C=C-Br
-CEC-C-C-
10)
11)
F Br
i
OH
مله
12)
Br
i
13)
14)
15)
CH3CHFCHFC=CH
C(OH)Br2CHF(CH2)4CH2CH3
CH3(CH2)3CH=CH(CH2)2CH3
Name
1) 3-fluoro, 1-butene
2) 2-heptene
2,3-difluoro-
1-pentene
4) 6-iodo,4-methyl-
2-decyne
5) 4,4-dibromo-
1,2-butandiol
Complete structural formula
F
-C=C-C-C-
Line formula
Condensed structural formula
N
F
CH2=CHCHFCH3
1.
Part 1: Naming Organic Compounds
он
H₁C-C-CH3
CH3
Br
CI CI
2. Br-CH-CH-CH₂
H₂C-CH-C= -CH-CH2-CH3
3.
HC-CH-CH-C-OH
5. H₂C-CH-CH₂-OH
7.
OH
4.
CH
CH₂-CH₂
6.
сно
CH-CH-CH-CH₂-CH₂
H₁₂C-CH-CH-CH-CH₁₂-CH₁₂
8.
OH
Chapter 2 Solutions
CHEMISTRY:PRIN.+REACTIONS-OWLV2 ACCESS
Ch. 2 - Atomic Theory and Laws State in your own words the...Ch. 2 - State in your own words the law of constant...Ch. 2 - Two basic laws of chemistry are the law of...Ch. 2 - Two basic laws of chemistry are the law of...Ch. 2 - Who discovered the electron? Describe the...Ch. 2 - Who discovered the nucleus? Describe the...Ch. 2 - Selenium is widely sold as a dietary supplement....Ch. 2 - Radon is a radioactive gas that can cause lung...Ch. 2 - How do the isotopes of argon, Ar-36, Ar-38, and...Ch. 2 - Consider two isotopes Fe-54 and Fe-56. (a) Write...
Ch. 2 - Uranium-235 is the isotope of uranium commonly...Ch. 2 - An isotope of americium (Am) with 146 neutrons is...Ch. 2 - Prob. 13QAPCh. 2 - Prob. 14QAPCh. 2 - Prob. 15QAPCh. 2 - See the definition for isobars in Question 15....Ch. 2 - Calculate the mass ratio of a bromine atom to an...Ch. 2 - Arrange the following in the order of increasing...Ch. 2 - Cerium is the most abundant rare earth metal. Pure...Ch. 2 - Consider the three stable isotopes of oxygen with...Ch. 2 - Bromine has two occuring isotopes: 79Br with...Ch. 2 - Rubidium has two naturally occurring isotopes:...Ch. 2 - Strontium has four isotopes with the following...Ch. 2 - Neon is an inert gas with three stable isotopes....Ch. 2 - Naturally occurring silver (Ag) consists of two...Ch. 2 - Copper has two naturally occurring isotopes. Cu-63...Ch. 2 - Silicon (averageatomicmass=28.0855amu) has three...Ch. 2 - Magnesium (averageatomicmass=24.305amu) consists...Ch. 2 - Zinc has four stable isotopes: Zn-64, Zn-66,...Ch. 2 - Chlorine has two isotopes, Cl-35 and Cl-37. Their...Ch. 2 - Lead is a heavy metal that remains in the...Ch. 2 - Silversmiths are warned to limit their exposure to...Ch. 2 - Determine (a) the number of atoms in 0.185 g of...Ch. 2 - For bismuth (Bi), determine (a) the number of...Ch. 2 - The isotope Si-28 has a mass of 27.977 amu. For...Ch. 2 - Myocardial perfusion imaging (MPI) is the latest...Ch. 2 - A cube of sodium has length 1.25 in. How many...Ch. 2 - A cylindrical piece of pure copper (d=8.92g/cm2)...Ch. 2 - Give the symbols for (a) potassium (b) cadmium (c)...Ch. 2 - Prob. 40QAPCh. 2 - Prob. 41QAPCh. 2 - Prob. 42QAPCh. 2 - How many metals are in the following groups? (a)...Ch. 2 - How many nonmetals are in the following periods?...Ch. 2 - Which group in the periodic table (a) has one...Ch. 2 - Which period of the periodic table (a) has no...Ch. 2 - Prob. 47QAPCh. 2 - Prob. 48QAPCh. 2 - Prob. 49QAPCh. 2 - Prob. 50QAPCh. 2 - Prob. 51QAPCh. 2 - Complete the table given below.Ch. 2 - Classify the following compounds as electrolytes...Ch. 2 - Prob. 54QAPCh. 2 - Prob. 55QAPCh. 2 - Prob. 56QAPCh. 2 - Prob. 57QAPCh. 2 - Write the names of the following molecules. (a)...Ch. 2 - Prob. 59QAPCh. 2 - Prob. 60QAPCh. 2 - Prob. 61QAPCh. 2 - Prob. 62QAPCh. 2 - Prob. 63QAPCh. 2 - Prob. 64QAPCh. 2 - Write the names of the following ionic compounds....Ch. 2 - Prob. 66QAPCh. 2 - Complete the following table.Ch. 2 - Complete the following table.Ch. 2 - Prob. 69QAPCh. 2 - Prob. 70QAPCh. 2 - Prob. 71QAPCh. 2 - Prob. 72QAPCh. 2 - Criticize each of the following statements: (a)...Ch. 2 - Which of the following statements is/are always...Ch. 2 - Some brands of salami contain 0.090% sodium...Ch. 2 - Carbon tetrachloride, CCl4, was a popular...Ch. 2 - Prob. 77QAPCh. 2 - Prob. 78QAPCh. 2 - Prob. 79QAPCh. 2 - Use the law of conservation of mass to determine...Ch. 2 - Prob. 81QAPCh. 2 - Prob. 82QAPCh. 2 - Scientists are trying to synthesize elements with...Ch. 2 - Write the nuclear symbol for the element whose...Ch. 2 - Prob. 85QAPCh. 2 - Write the atomic symbol for the element whose ion...Ch. 2 - Prob. 87QAPCh. 2 - Three compounds containing only carbon and...Ch. 2 - Ethane and ethylene are two gases containing only...Ch. 2 - Calculate the average density of a single Al-27...Ch. 2 - Prob. 91QAPCh. 2 - Each time you inhale, you take in about 500 mL...Ch. 2 - Hydrogen gas is prepared in a lab experiment. In...
Knowledge Booster
Similar questions
- 11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardAssign these COSY Spectrumarrow_forward
- Assign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forward
- Predict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forward
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning