
(a)
Interpretation:
To write the molecular formula and condensed formula of given molecule:
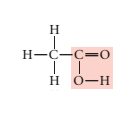
Concept introduction:
Condensed formula: It is a way of writing the molecule where all the atoms are arranged in order as appear in structural formula but while writing condensed formula, bond lines are omitted. For example: Condensed formula of propane is CH3 CH2 CH3.
Molecular formula: It is the easiest way of representing the number of atoms of each element in a molecule.
While writing molecule formula,
Answer to Problem 48QAP
Condensed formula: CH3 COOH
Molecular formula: C2 H4 O2
Explanation of Solution
Given structural formula-
In above structural formula, CH3 group is attached with acetic acid group through carbon atom. Hence, its condensed formula will be CH3 COOH.
In 1 molecule of CH3 COOH, there are 2 carbon atoms, 4 hydrogen atoms and 2 oxygen atoms. Therefore, its molecular formula is C2 H4 O2.
(b)
Interpretation:
To write the molecular formula and condensed formula of given molecule:
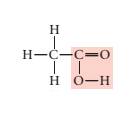
Concept introduction:
Condensed formula: It is a way of writing the molecule where all the atoms are arranged in order as appear in structural formula but while writing condensed formula, bond lines are omitted. For example: Condensed formula of propane is CH3 CH2 CH3.
Molecular formula: It is the easiest way of representing the number of atoms of each element in a molecule.
While writing molecule formula, symbol of each element is written with their respective number of atoms in a single molecule. And these numbers are written in subscript as:
Answer to Problem 48QAP
Condensed formula: CH3 Cl
Molecular formula: CH3 Cl
Explanation of Solution
Given structural formula:
In above structural formula, CH3 group is attached with Chlorine group through carbon atom. Hence, its condensed formula will be CH3 Cl.
In 1 molecule of CH3 Cl, there are 1 carbon atom, 3 hydrogen atoms and 1 chlorine atom. Therefore, its molecular formula is CH3 Cl.
Want to see more full solutions like this?
Chapter 2 Solutions
PRINCIPLES+REACTIONS
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





