
(a)
Interpretation:
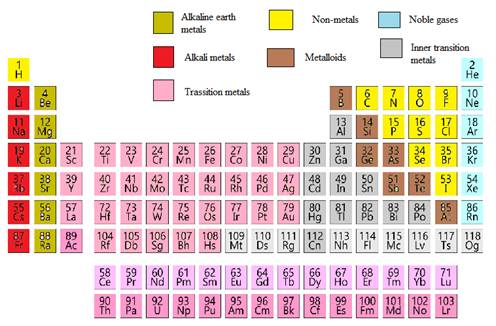
The potassium element needs to be classified as metals, non-metals,
Concept introduction:
In the modern periodic table, all the known elements are arranged in organized tabular manner (having groups and periods) based on their
Elements are also classified based on metal, non-metals, transition element and post- transition elements in modern periodic table.
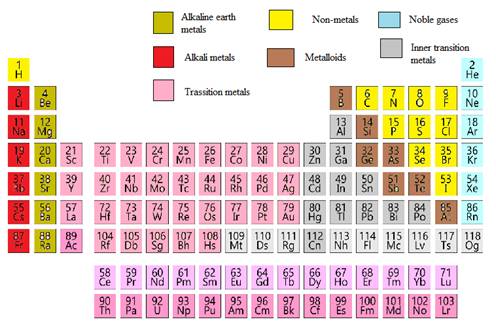
Modern periodic table is represented as follows:
(b)
Interpretation:
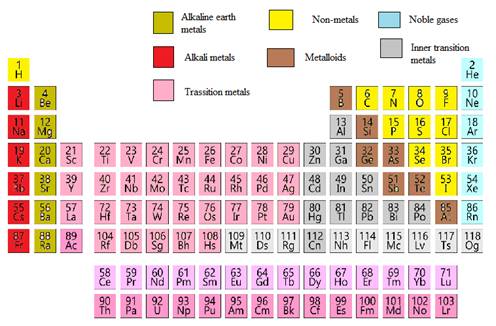
The cadmium element needs to be classified as metals, non-metals, transition elements or post transition elements.
Concept introduction:
In the modern periodic table, all the known elements are arranged in organized tabular manner (having groups and periods) based on their atomic number. In this table, atomic number, chemical symbol with its chemical name and average atomic mass is written.
Elements are also classified based on metal, non-metals, transition element and post- transition elements in modern periodic table.
Modern periodic table is represented as follows:
(c)
Interpretation:
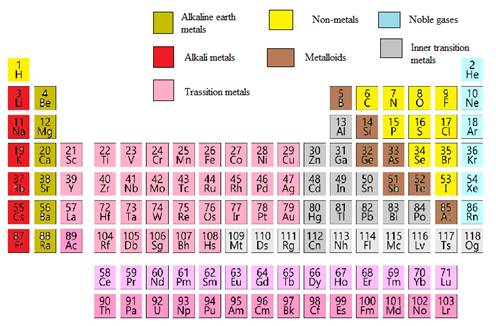
The aluminum element needs to be classified as metals, non-metals, transition elements or post transition elements.
Concept introduction:
In the modern periodic table, all the known elements are arranged in organized tabular manner (having groups and periods) based on their atomic number. In this table, atomic number, chemical symbol with its chemical name and average atomic mass is written.
Elements are also classified based on metal, non-metals, transition element and post- transition elements in modern periodic table.
Modern periodic table is represented as follows:
(d)
Interpretation:
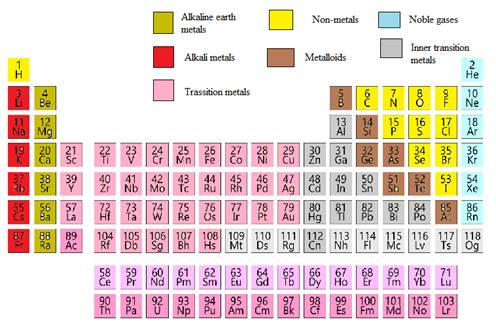
The antimony element needs to be classified as metals, non-metals, transition elements or post transition elements.
Concept introduction:
In the modern periodic table, all the known elements are arranged in organized tabular manner (having groups and periods) based on their atomic number. In this table, atomic number, chemical symbol with its chemical name and average atomic mass is written.
Elements are also classified based on metal, non-metals, transition element and post- transition elements in modern periodic table.
Modern periodic table is represented as follows:
(e)
Interpretation:
The phosphorus element needs to be classified as metals, non-metals, transition elements or post transition elements.
Concept introduction:
In the modern periodic table, all the known elements are arranged in organized tabular manner (having groups and periods) based on their atomic number. In this table, atomic number, chemical symbol with its chemical name and average atomic mass is written.
Elements are also classified based on metal, non-metals, transition element and post- transition elements in modern periodic table.
Modern periodic table is represented as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
PRINCIPLES+REACTIONS
- The following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forward
- Which one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forward
- Ppplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





