
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 2.90AP
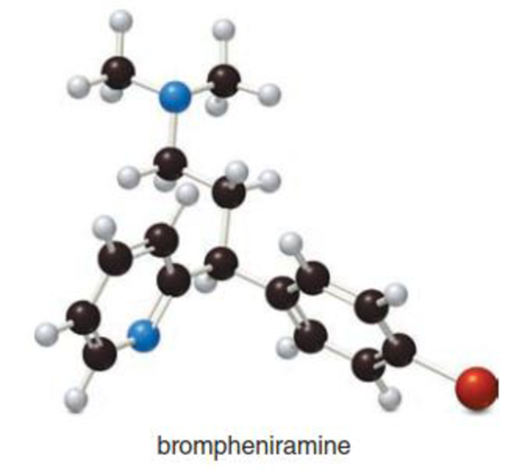
- (a) What is the chemical formula for brompheniramine, an antihistamine used to relieve the runny nose and sneezing associated with allergies? (b) Which element in brompheniramine has valence electrons that are farthest from
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(b) A new element, "X", is discovered and found to have 2 electrons in its outer level.
Is X a metal or non-metal? Predict the formula its ion would have in any ionic
compounds it forms.
How many inner, outer, and valence electrons are present in an atom of each of the following elements?(a) O(b) Sn(c) Ca(d) Fe(e) Se
Fig 12.1 represents a neutral lithium atom. All the particles in the atom are shown on the diagram.
(a)
Use Fig. 12.1 to help you answer the following questions.
(1)
How many electrons does this atom have?
(ii)
What is the value of the proton number of this atom?
(ii)
How many neutrons does the atom have?
(iv)
What is the value of the nucleon number of this atom?
(b)
Write the appropriate numbers in the boxes below, to represent this atom of Lithium in
nuclide notation.
Li
orbit
nucleus
Fig. 12.1
(c)
Lithium-6 is another isotope of lithium. It has 6nucleons.
(i) What is an isotope?
(ii) How many protons does Lithium-6 have?
(ii) How many neutrons does Lithium-6 have
Chapter 2 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 2.1 - Give the symbol for each element. a. calcium, a...Ch. 2.1 - Prob. 2.2PCh. 2.1 - Give the name corresponding to each element...Ch. 2.1 - Locate each element in the periodic table and...Ch. 2.1 - Classify each micronutrient in Figure 2.2 as a...Ch. 2.1 - Identify the elements used in each example of...Ch. 2.1 - Identify the elements in each chemical formula,...Ch. 2.1 - Prob. 2.8PCh. 2.2 - Prob. 2.9PCh. 2.2 - Prob. 2.10P
Ch. 2.2 - Prob. 2.11PCh. 2.2 - Prob. 2.12PCh. 2.2 - Prob. 2.13PCh. 2.3 - Prob. 2.14PCh. 2.3 - Prob. 2.15PCh. 2.3 - Prob. 2.16PCh. 2.3 - Prob. 2.17PCh. 2.3 - Prob. 2.18PCh. 2.4 - Prob. 2.19PCh. 2.4 - Give the period and group number for each element:...Ch. 2.4 - Prob. 2.21PCh. 2.4 - Prob. 2.22PCh. 2.5 - Prob. 2.23PCh. 2.6 - Prob. 2.24PCh. 2.6 - Prob. 2.25PCh. 2.6 - Prob. 2.26PCh. 2.7 - Identify the total number of electrons, the number...Ch. 2.7 - Prob. 2.28PCh. 2.7 - Prob. 2.29PCh. 2.8 - Which element in each pair has the larger atomic...Ch. 2.8 - Which element in each pair has the higher...Ch. 2.8 - Prob. 2.32PCh. 2 - Identify the elements used in each example of...Ch. 2 - Prob. 2.34UKCCh. 2 - Prob. 2.35UKCCh. 2 - Prob. 2.36UKCCh. 2 - Prob. 2.37UKCCh. 2 - Prob. 2.38UKCCh. 2 - Prob. 2.39UKCCh. 2 - Prob. 2.40UKCCh. 2 - Prob. 2.41UKCCh. 2 - Prob. 2.42UKCCh. 2 - Prob. 2.43UKCCh. 2 - Prob. 2.44UKCCh. 2 - Prob. 2.45APCh. 2 - Prob. 2.46APCh. 2 - Prob. 2.47APCh. 2 - Identify the elements in each chemical formula and...Ch. 2 - Prob. 2.49APCh. 2 - Prob. 2.50APCh. 2 - Prob. 2.51APCh. 2 - Prob. 2.52APCh. 2 - Prob. 2.53APCh. 2 - Prob. 2.54APCh. 2 - Prob. 2.55APCh. 2 - Prob. 2.56APCh. 2 - Prob. 2.57APCh. 2 - Prob. 2.58APCh. 2 - The most common isotope of oxygen has a mass...Ch. 2 - Prob. 2.60APCh. 2 - Prob. 2.61APCh. 2 - Prob. 2.62APCh. 2 - Prob. 2.63APCh. 2 - Prob. 2.64APCh. 2 - Prob. 2.65APCh. 2 - Prob. 2.66APCh. 2 - Prob. 2.67APCh. 2 - Prob. 2.68APCh. 2 - Prob. 2.69APCh. 2 - Prob. 2.70APCh. 2 - Prob. 2.71APCh. 2 - Prob. 2.72APCh. 2 - Prob. 2.73APCh. 2 - Prob. 2.74APCh. 2 - Prob. 2.75APCh. 2 - Prob. 2.76APCh. 2 - Prob. 2.77APCh. 2 - Prob. 2.78APCh. 2 - Prob. 2.79APCh. 2 - Prob. 2.80APCh. 2 - Prob. 2.81APCh. 2 - Prob. 2.82APCh. 2 - Prob. 2.83APCh. 2 - Arrange the elements in each group in order of...Ch. 2 - Prob. 2.85APCh. 2 - Prob. 2.86APCh. 2 - Answer the following questions about...Ch. 2 - Prob. 2.88APCh. 2 - Prob. 2.89APCh. 2 - (a) What is the chemical formula for...Ch. 2 - Prob. 2.91CPCh. 2 - Prob. 2.93BTCCh. 2 - Prob. 2.95BTC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the electron dot formula for each of the following elements: (a) Si (b)Xearrow_forwardArrange in order of increasing nonmetallic character. (Use the appropriate <, =, or > symbol to separate substances in the list.) (a) the Period 4 elements V, Ge, and K (b) the Group 5A elements N, As, and Bi Arrange in order of increasing atomic size. (Use the appropriate <, =, or > symbol to separate substances in the list.) (a) the Period 3 elements Mg, Si, and Ar (b) the Group 2A elements Ca, Ba, and Srarrow_forwardWhich of these elements is most likely to form ions with a 2+charge?(a) Li (b) Ca (c) O (d) P (e) Clarrow_forward
- Write the ground-state electron configuration for each atom. After each atom is its atomic number in parentheses. (a) Sodium (11) (b) Magnesium (12) (c) Oxygen (8) (d) Nitrogen (7)arrow_forwardAn oxygen atom has eight protons. (a) In the following diagram, sketch in the arrangement of electrons around the nucleus of the oxygen atom. (b) How many more electrons will it take to fill the outermost electron shell?arrow_forwardchoose three . What are the characteristerses of a metal like element? (a) they try to get additional electrons (b) they are melleable. (c) they are lightly to give away or share electrons. (d) they are good conductors of electricityarrow_forward
- Mn 2 + is an essential nutrient needed for blood clotting and the formation of the protein collagen. (a) How many protons and electrons are found in a neutral manganese atom? (b) How many electrons and protons are found in the cation Mn 2 +? (c) Write the electronic confi guration of the element manganese and suggest which electrons are lost to form the Mn 2 + cation.arrow_forwardvi. Answer true or false. (a) Elements in the same column of the Periodic Table have the same outer-shell electron configuration. (b) All Group 1A elements have one electron in their valence shell. (c) All Group 6A elements have six electrons in their valence shell. (d) All Group 8A elements have eight electrons in their valence shell. (e) Period 1 of the Periodic Table has one element, period 2 has two elements, period 3 has three elements, and so forth. (1) Period 2 results from filling the 2s and 2p orbitals and, therefore, there are eight elements in period 2. (g) Period 3 results from filling the 3s, 3p, and 3d orbitals and, therefore, there are nine elements in period 3. (h) The main-group elements are s block and p block elements.arrow_forwardUsing Lewis electron-dot symbols to depict the monatomic ions formed from each of the following reactants, predict the formula of the compound the ions produce.(Type your answer using the format CO2 for CO2.) (a) O and Ca (b) N and Mg (c) Br and Li (d) K and Parrow_forward
- Zinc is an essential nutrient needed by many enzymes to maintain proper cellular function. Zinc is obtained in many dietary sources, including oysters, beans, nuts, whole grains, and sunfl ower seeds. (a) How many protons and electrons are found in a neutral zinc atom? (b) How many electrons and protons are found in the Zn 2 + cation? (c) Write the electronic confi guration of the element zinc, and suggest which electrons are lost to form the Zn 2 + cation.arrow_forwardWrite the chemical formulas for the following compounds:(a) Silver cyanide(b) Calcium hypochlorite(c) Potassium chromate(d) Gallium oxide(e) Potassium superoxide(f) Barium hydrogen carbonatearrow_forwardQuestion A11 (a) Write the full electronic configuration of Cu, using s,p,d,f notation. (b) Complete the table below: Atom/Ion 64 Ni2+ 28 Proton Neutrons Electrons (c) Predict whether Na or Cl has the more stable 1s² shell and explain your rationale. (d) What three factors can influence the Ionization energy of group 2 elements?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Liquids: Crash Course Chemistry #26; Author: Crash Course;https://www.youtube.com/watch?v=BqQJPCdmIp8;License: Standard YouTube License, CC-BY
Chemistry of Group 16 elements; Author: Ch-11 Chemical Engg, Chemistry and others;https://www.youtube.com/watch?v=5B1F0aDgL6s;License: Standard YouTube License, CC-BY