
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Question
Chapter 2, Problem 2.34UKC
(a)
Interpretation Introduction
Interpretation:
The elements used in the example a has to be given.
The molecular art is,

Figure 1
(b)
Interpretation Introduction
Interpretation:
The element used in the example b has to be given.
The molecular art is,
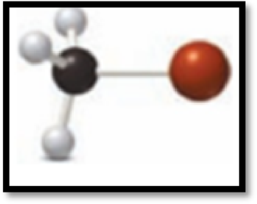
Figure 2
(c)
Interpretation Introduction
Interpretation:
The element used in the example c has to be given.
The ball and stick representation in example c is,
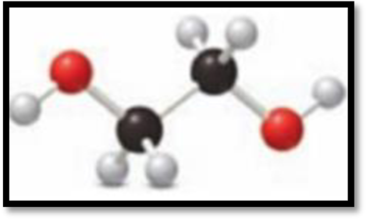
Figure 3
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
R₂
R₁
R₁
a
R
Rg
Nu
R₂
Rg
R₁
R
R₁₂
R3
R
R
Nu enolate forming
R₁ R
B-Alkylated carbonyl
species or amines
Cyclic B-Ketoester
R₁₁
HOB
R
R₁B
R
R₁₂
B-Hydroxy carbonyl
R
diester
R2 R3
R₁
RB
OR
R₂ 0
aB-Unsaturated carbonyl
NaOR
Aldol
HOR
reaction
1) LDA
2) R-X
3) H₂O/H₂O
ketone,
aldehyde
1) 2°-amine
2) acid chloride
3) H₂O'/H₂O
0
O
R₁
R₁
R
R₁
R₁₂
Alkylated a-carbon
R₁
H.C
R₁
H.C
Alkylated methyl ketone
acetoacetic
ester
B-Ketoester
ester
R₁
HO
R₂ R
B-Dicarbonyl
HO
Alkylated carboxylic acid
malonic ester
Write the reagents required to bring about each reaction next to the arrows shown.
Next, record any regiochemistry or stereochemistry considerations relevant to the
reaction. You should also record any key aspects of the mechanism, such as forma-
tion of an important intermediate, as a helpful reminder. You may want to keep
track of all reactions that make carbon-carbon bonds, because these help you build
large molecules from smaller fragments. This especially applies to the reactions in…
Provide the reasonable steps to achieve the following synthesis.
Identify which compound is more acidic. Justify your choice.
Chapter 2 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 2.1 - Give the symbol for each element. a. calcium, a...Ch. 2.1 - Prob. 2.2PCh. 2.1 - Give the name corresponding to each element...Ch. 2.1 - Locate each element in the periodic table and...Ch. 2.1 - Classify each micronutrient in Figure 2.2 as a...Ch. 2.1 - Identify the elements used in each example of...Ch. 2.1 - Identify the elements in each chemical formula,...Ch. 2.1 - Prob. 2.8PCh. 2.2 - Prob. 2.9PCh. 2.2 - Prob. 2.10P
Ch. 2.2 - Prob. 2.11PCh. 2.2 - Prob. 2.12PCh. 2.2 - Prob. 2.13PCh. 2.3 - Prob. 2.14PCh. 2.3 - Prob. 2.15PCh. 2.3 - Prob. 2.16PCh. 2.3 - Prob. 2.17PCh. 2.3 - Prob. 2.18PCh. 2.4 - Prob. 2.19PCh. 2.4 - Give the period and group number for each element:...Ch. 2.4 - Prob. 2.21PCh. 2.4 - Prob. 2.22PCh. 2.5 - Prob. 2.23PCh. 2.6 - Prob. 2.24PCh. 2.6 - Prob. 2.25PCh. 2.6 - Prob. 2.26PCh. 2.7 - Identify the total number of electrons, the number...Ch. 2.7 - Prob. 2.28PCh. 2.7 - Prob. 2.29PCh. 2.8 - Which element in each pair has the larger atomic...Ch. 2.8 - Which element in each pair has the higher...Ch. 2.8 - Prob. 2.32PCh. 2 - Identify the elements used in each example of...Ch. 2 - Prob. 2.34UKCCh. 2 - Prob. 2.35UKCCh. 2 - Prob. 2.36UKCCh. 2 - Prob. 2.37UKCCh. 2 - Prob. 2.38UKCCh. 2 - Prob. 2.39UKCCh. 2 - Prob. 2.40UKCCh. 2 - Prob. 2.41UKCCh. 2 - Prob. 2.42UKCCh. 2 - Prob. 2.43UKCCh. 2 - Prob. 2.44UKCCh. 2 - Prob. 2.45APCh. 2 - Prob. 2.46APCh. 2 - Prob. 2.47APCh. 2 - Identify the elements in each chemical formula and...Ch. 2 - Prob. 2.49APCh. 2 - Prob. 2.50APCh. 2 - Prob. 2.51APCh. 2 - Prob. 2.52APCh. 2 - Prob. 2.53APCh. 2 - Prob. 2.54APCh. 2 - Prob. 2.55APCh. 2 - Prob. 2.56APCh. 2 - Prob. 2.57APCh. 2 - Prob. 2.58APCh. 2 - The most common isotope of oxygen has a mass...Ch. 2 - Prob. 2.60APCh. 2 - Prob. 2.61APCh. 2 - Prob. 2.62APCh. 2 - Prob. 2.63APCh. 2 - Prob. 2.64APCh. 2 - Prob. 2.65APCh. 2 - Prob. 2.66APCh. 2 - Prob. 2.67APCh. 2 - Prob. 2.68APCh. 2 - Prob. 2.69APCh. 2 - Prob. 2.70APCh. 2 - Prob. 2.71APCh. 2 - Prob. 2.72APCh. 2 - Prob. 2.73APCh. 2 - Prob. 2.74APCh. 2 - Prob. 2.75APCh. 2 - Prob. 2.76APCh. 2 - Prob. 2.77APCh. 2 - Prob. 2.78APCh. 2 - Prob. 2.79APCh. 2 - Prob. 2.80APCh. 2 - Prob. 2.81APCh. 2 - Prob. 2.82APCh. 2 - Prob. 2.83APCh. 2 - Arrange the elements in each group in order of...Ch. 2 - Prob. 2.85APCh. 2 - Prob. 2.86APCh. 2 - Answer the following questions about...Ch. 2 - Prob. 2.88APCh. 2 - Prob. 2.89APCh. 2 - (a) What is the chemical formula for...Ch. 2 - Prob. 2.91CPCh. 2 - Prob. 2.93BTCCh. 2 - Prob. 2.95BTC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the reasonable steps to achieve the following synthesis.arrow_forwardWhen anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forward
- For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardConsider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forward
- For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardGiven the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY