
Concept explainers
(a)
Interpretation:
The ground state electronic configuration of silicon has to be given.
Concept Introduction:
The distribution of electrons in various orbitals is known as electronic configuration.
The filling of orbitals in the ground state is determined by the following rule:
Aufbau Principle:
The Aufbau principle states that in the ground state of an atom the orbital with lower energy are filled up first before the filling of the orbital with ah higher energy commences.
(b)
Interpretation:
The valence electrons of silicon have to be given.
Concept Introduction:
Valence electrons:
Valence electrons are electrons that exist in the outermost shell surrounding an atomic nucleus.
(c)
Interpretation:
The electron dot structure of silicon has to be given.
Concept Introduction:
Electron dot structure:
A Lewis dot diagram is a representation of the valence electrons of an atom that uses dots around the
Example:
The valence electron of Mg is two it can be represented in electron dot structure as,
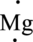
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Principles of General, Organic, Biological Chemistry
- Don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forward
- Briefly indicate the structure and bonding of silicates.arrow_forward4 Part C Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma.arrow_forwardTry: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
