
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
7th Edition
ISBN: 9781305866966
Author: STOKER
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.23EP
Consider the following rulers as instruments for the measurement of length.
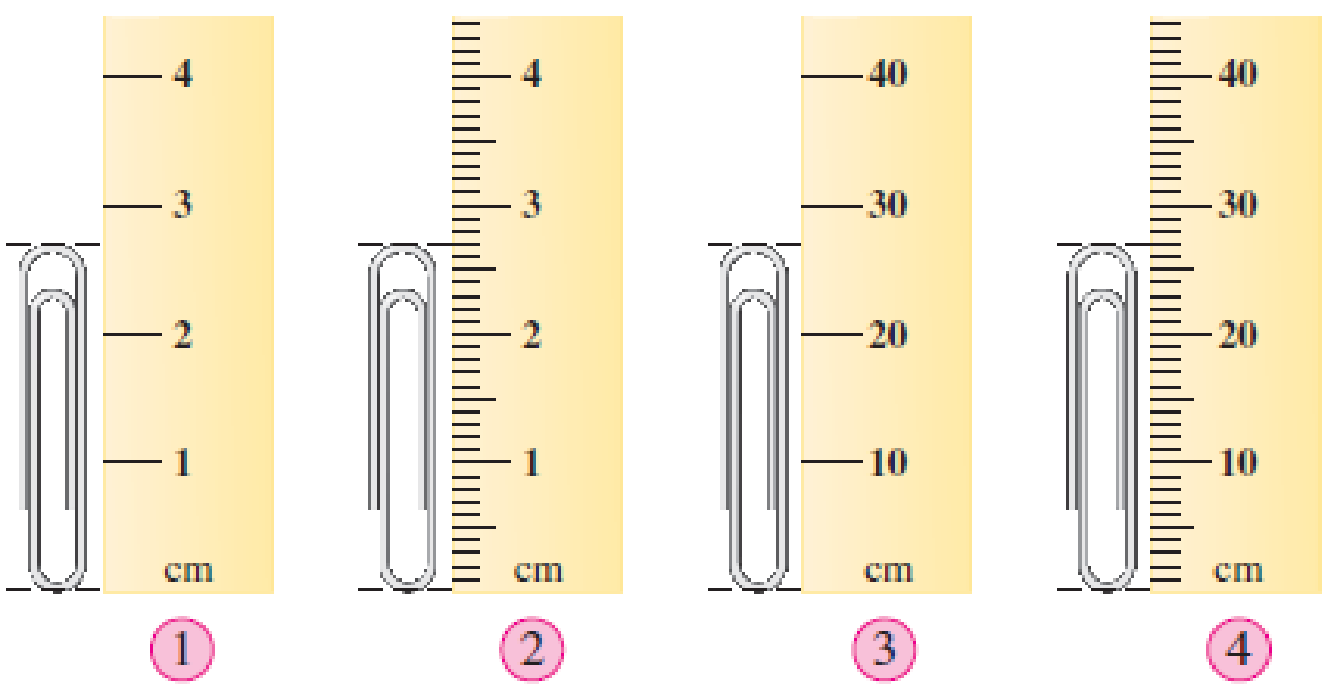
What would the uncertainty be in measurements made using the following?
- a. Ruler 1
- b. Ruler 4
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
It is possible to make a perfectly precise measurement?Explain.
You wish to quantify your favourite compound in an experimental solution using
spectrophotometry. First, you use standard solutions of your favourite compound to
collect the following standard curve data. Calculate the slope of the line of best fit
to two decimal places, and enter it in the box below the table.
Standard curve data
Conc (MM)
0.300
0.600
0.900
1.200
1.500
Abs
0.225
0.450
0.675
0.900
1.125
Next, you prepare an experimental sample by mixing 0.25 mL of the compound
solution with 9.75 mL of distilled water. Calculate the dilution factor and enter it as a
whole number (no decimals).
Next, you read an absorbance of 0.72 absorbance units for the diluted sample.
Calculate the concentration (mm) of the original solution of the compound. Round
this answer to one decimal place.
Previous Page
Next Page
MacBook Air
Page 5 of 13
Which pharmaceutical products need specific gravity measurement? Mention three examples
Chapter 2 Solutions
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
Ch. 2.1 - Prob. 1QQCh. 2.1 - Preference by scientists for metric system unit...Ch. 2.2 - In which of the following pairings of metric...Ch. 2.2 - In which of the following sequences are the metric...Ch. 2.2 - Which of the following is a correct pairing of...Ch. 2.2 - Prob. 4QQCh. 2.2 - Prob. 5QQCh. 2.2 - Prob. 6QQCh. 2.2 - Prob. 7QQCh. 2.3 - Prob. 1QQ
Ch. 2.3 - Prob. 2QQCh. 2.4 - Prob. 1QQCh. 2.4 - Prob. 2QQCh. 2.4 - Prob. 3QQCh. 2.4 - Prob. 4QQCh. 2.4 - Prob. 5QQCh. 2.4 - Prob. 6QQCh. 2.5 - In which of the following cases is the given...Ch. 2.5 - When rounded to three significant figures, the...Ch. 2.5 - Prob. 3QQCh. 2.5 - Prob. 4QQCh. 2.6 - Prob. 1QQCh. 2.6 - Prob. 2QQCh. 2.6 - Prob. 3QQCh. 2.6 - Prob. 4QQCh. 2.6 - Prob. 5QQCh. 2.6 - Prob. 6QQCh. 2.7 - Prob. 1QQCh. 2.7 - Prob. 2QQCh. 2.7 - Which of the following is an incorrect conversion...Ch. 2.7 - Prob. 4QQCh. 2.8 - Prob. 1QQCh. 2.8 - Prob. 2QQCh. 2.9 - Prob. 1QQCh. 2.9 - Prob. 2QQCh. 2.9 - Prob. 3QQCh. 2.9 - What is the mass, in grams, of 30.0 mL of liquid...Ch. 2.10 - The freezing point of water is a. 0F b. 0 K c. 0C...Ch. 2.10 - Prob. 2QQCh. 2.10 - Prob. 3QQCh. 2.10 - Prob. 4QQCh. 2 - What is the main reason scientists prefer to use...Ch. 2 - List the more common types of measurements made in...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Arrange each of the following from smallest to...Ch. 2 - Arrange each of the following from smallest to...Ch. 2 - Which of the two given units is the more logical...Ch. 2 - Which of the two given units is the more logical...Ch. 2 - A person is told that there are 60 minutes in an...Ch. 2 - Prob. 2.12EPCh. 2 - Indicate whether the number in each of the...Ch. 2 - Indicate whether the number in each of the...Ch. 2 - Indicate whether each of the following quantities...Ch. 2 - Indicate whether each of the following quantities...Ch. 2 - Identify the estimated digit in each of the...Ch. 2 - Identify the estimated digit in each of the...Ch. 2 - Prob. 2.19EPCh. 2 - Prob. 2.20EPCh. 2 - Indicate to what decimal position readings should...Ch. 2 - Indicate to what decimal position readings should...Ch. 2 - Consider the following rulers as instruments for...Ch. 2 - Consider the following rulers as instruments for...Ch. 2 - Using the rulers given in Problem 2-23, what is...Ch. 2 - Using the rulers given in Problem 2-23, what is...Ch. 2 - With which of the rulers in Problem 2-23 was each...Ch. 2 - With which of the rulers in Problem 2-23 was each...Ch. 2 - Determine the number of significant figures in...Ch. 2 - Determine the number of significant figures in...Ch. 2 - Determine the number of significant figures in...Ch. 2 - Determine the number of significant figures in...Ch. 2 - In which of the following pairs of numbers do both...Ch. 2 - In which of the following pairs of numbers do both...Ch. 2 - Prob. 2.35EPCh. 2 - In the pairs of numbers of Problem 2-34, tell...Ch. 2 - Prob. 2.37EPCh. 2 - Complete the following table by filling in the...Ch. 2 - Prob. 2.39EPCh. 2 - The number of people present at an outdoor rock...Ch. 2 - Round off each of the following numbers to the...Ch. 2 - Round off each of the following numbers to the...Ch. 2 - Prob. 2.43EPCh. 2 - Round off (or add zeros) to each of the following...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Without actually solving, indicate the number of...Ch. 2 - Without actually solving, indicate the number of...Ch. 2 - Prob. 2.49EPCh. 2 - Carry out the following multiplications and...Ch. 2 - Carry out the following additions and...Ch. 2 - Carry out the following additions and...Ch. 2 - What is the uncertainty in the measured value...Ch. 2 - What is the uncertainty in the measured value...Ch. 2 - For each of the following numbers, will the...Ch. 2 - For each of the following numbers, will the...Ch. 2 - Prob. 2.57EPCh. 2 - Prob. 2.58EPCh. 2 - Prob. 2.59EPCh. 2 - For each of the numbers in Problem 2-56, how many...Ch. 2 - Express the following measured values in...Ch. 2 - Express the following measured values in...Ch. 2 - Change each of the following measured values from...Ch. 2 - Change each of the following measured values from...Ch. 2 - Prob. 2.65EPCh. 2 - Prob. 2.66EPCh. 2 - What is the uncertainty, in terms of a power of...Ch. 2 - What is the uncertainty, in terms of a power of...Ch. 2 - Write each of the following numbers in scientific...Ch. 2 - Write each of the following numbers in scientific...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Prob. 2.75EPCh. 2 - Indicate whether each of the following equations...Ch. 2 - Using dimensional analysis, convert each of the...Ch. 2 - Using dimensional analysis, convert each of the...Ch. 2 - The human stomach produces approximately 2500 mL...Ch. 2 - A typical loss of water through sweating for a...Ch. 2 - The mass of premature babies is customarily...Ch. 2 - The smallest bone in the human body, which is in...Ch. 2 - What volume of water, in gallons, would be...Ch. 2 - What volume of gasoline, in milliliters, would be...Ch. 2 - An individual weighs 83.2 kg and is 1.92 m tall....Ch. 2 - An individual weighs 135 lb and is 5 ft 4 in....Ch. 2 - Prob. 2.87EPCh. 2 - Prob. 2.88EPCh. 2 - Prob. 2.89EPCh. 2 - When each of the following measurements of mass is...Ch. 2 - A sample of mercury is found to have a mass of...Ch. 2 - A sample of sand is found to have a mass of 12.0 g...Ch. 2 - Acetone, the solvent in nail polish remover, has a...Ch. 2 - Silver metal has a density of 10.40 g/cm3. What is...Ch. 2 - The density of homogenized milk is 1.03 g/mL. How...Ch. 2 - Nickel metal has a density of 8.90 g/cm3. How much...Ch. 2 - Water has a density of 1.0 g/cm3 at room...Ch. 2 - Air has a density of 1.29 g/L at room temperature....Ch. 2 - Prob. 2.99EPCh. 2 - A two-gram sample of a red-colored liquid is found...Ch. 2 - Calculate the volume, in milliliters, for each of...Ch. 2 - Calculate the volume, in milliliters, for each of...Ch. 2 - An oven for baking pizza operates at approximately...Ch. 2 - A comfortable temperature for bathtub water is...Ch. 2 - Mercury freezes at 38.9C. What is the coldest...Ch. 2 - Prob. 2.106EPCh. 2 - Prob. 2.107EPCh. 2 - Which is the higher temperature, 15C or 4F?
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Now calculate the slope and equation for the best fit line. You may use excelarrow_forward* You can keep extra decimal places through most of this problem, but round your final estimate to one decimal place* You wish to quantify your favourite compound in an experimental solution using spectrophotometry. First, you use standard solutions of your favourite compound to collect the following standard curve data. Calculate the slope of the line of best fit. Standard curve data Conc (mM) 0.300 0.600 0.900 1.200 1.500 Abs 0.150 0.300 0.450 0.600 0.750 Next, you prepare an experimental sample by mixing 0.50 mL of the compound solution with 9.50 mL of distilled water, and you read an absorbance of 0.625 for this sample. Calculate the concentration (mM) of the original solution of compound. Round this answer to one decimal place.arrow_forwardE. 1) Define Accuracy of measurement Vs. Precision in a measurement.arrow_forward
- In the spectrofluorometric analysis of quinine, which of the following statements/phrases can be considered a factor level? A. Concentration of 2 ppm of iodide B. Concentration of quenchers C. The use of iodide D. Use of Quenchersarrow_forwardWhat is an advantage and disadvantage of the Thorpe flowmeter and the Bourdon Gauge flowmeter?arrow_forwardTo estimate drug dosages, doctors use a patient’s body surface area (BSA)(in meters squared) using the formula BSA = √hm/60, where h is the height in centimeters and m is the mass in kilograms. Calculate the rate of change of BSA with respect to height for a person of constant mass m = 80 kg. Express answer in correct units.arrow_forward
- An investigation was set up to determine the effect of various surfaces on the amount of time it takes a snail to move between two points. A ramp was made, covered with a piece of glass, and placed on a table at a 25-degree angle. Two lines, 15 centimeters apart, were drawn on the glass. A snail was placed on the upper line, and a stopwatch was used to measure the 2) amount of time it took the snail to move to the lower line. The results were recorded in a data table. The investigation was repeated using sandpaper and cardboard on the surface of the ramp. What must be done to show that the results of this investigation are valid? A) The ramp must be replaced with a log found in the habitat of the snail. B) The angle of the ramp must be increased to 35 degrees. C) The investigation must be repeated several times. D) The times in the data table must be averaged.arrow_forwardsee image belowarrow_forwardFurosemide is packaged as 10 mg/mL. A pharmacy technician must make a preparation totaling 25 mL of 2 mg/mL concentration. How much drug and how much diluent are used? (Textbook says use %w/v to calculate and the answer key says 5 mL concentrate; 20 mL diluent. I'm looking for how to set up and solve this problem.)arrow_forward
- Give a specific example on how to use spectrofluorometer in Cosmetic Science. Provide a procedure on how to do this example that you provided. Explain the procedure and what data would you gather?arrow_forwardCalculate the volume of distribution for a 70-kilogram man for one of the four drugs listed in the table below. Using your calculations, interpret the implications that can be inferred from the value you calculated. In your post, include your calculations and interpretation. Drug Drug A Drug B Drug C Drug D Volume of Distribution (L/kg) 16.0 + 4 1.1 +0.2 0.07 + 0.02 Volume of Distribution 0.23 + 0.09arrow_forwardYou have carried out an experiment using the spectrophotometry concept. A solutioncontaining compound X is mixed with reagent 1 and then reagent 2. This mixture produces ablue colour whose absorbance (A) could be read at 550 nm. The results are shown below. If the standard solution (compound X) used have a concentration of 1 mM:1. Calculate the quantity of the standard compound X in μmoles for each test tube (1-7).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Nutrition Through The Life CycleHealth & NutritionISBN:9781337919333Author:Brown, Judith E.Publisher:Cengage Learning,
Nutrition Through The Life CycleHealth & NutritionISBN:9781337919333Author:Brown, Judith E.Publisher:Cengage Learning,

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning





Nutrition Through The Life Cycle
Health & Nutrition
ISBN:9781337919333
Author:Brown, Judith E.
Publisher:Cengage Learning,
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY