
Microbiology with Diseases by Body System (5th Edition)
5th Edition
ISBN: 9780134477206
Author: Robert W. Bauman Ph.D.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 1VI
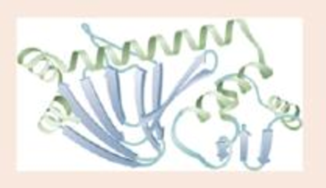
Label a portion of the molecule below; label two types of secondary structure; circle the tertiary structure.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following best describes why it is difficult to develop antiviral drugs? Explain why.
A. antiviral drugs are very difficult to develop andhave no side effects
B. viruses are difficult to target because they usethe host cell’s enzymes and ribosomes tometabolize and replicate
C. viruses are too small to be targeted by drugs
D. viral infections usually clear up on their ownwith no problems
This question has 3 parts (A, B, & C), and is under the subject of Nutrition. Thank you!
They got this question wrong the 2 previous times I uploaded it here, please make sure it's correvct this time.
Chapter 2 Solutions
Microbiology with Diseases by Body System (5th Edition)
Ch. 2 - Electrons zip around the nucleus at about 5...Ch. 2 - Chlorine and potassium atoms form ionic bonds,...Ch. 2 - Why are decomposition reactions exothermic, that...Ch. 2 - Why does the neutralization of an acid by a base...Ch. 2 - Raw Oysters and Antacids: A Deadly Mix? The highly...Ch. 2 - Why do the cell membranes of microbes living in...Ch. 2 - Prob. 1MCCh. 2 - The atomic mass of an atom most closely...Ch. 2 - One isotope of iodine differs from another in...Ch. 2 - Prob. 4MC
Ch. 2 - Which of the following terms most correctly...Ch. 2 - In water, cations and anions of salts dissociate...Ch. 2 - Prob. 7MCCh. 2 - Which of the following statements about a...Ch. 2 - Proteins are polymers of ___________. a. amino...Ch. 2 - Which of the following are hydrophobic organic...Ch. 2 - Fill in the Blanks 1. The outermost electron shell...Ch. 2 - Fill in the Blanks 2. The type of chemical bond...Ch. 2 - Prob. 3FIBCh. 2 - Prob. 4FIBCh. 2 - Fill in the Blanks 5. Groups of atoms such as NH2...Ch. 2 - Fill in the Blanks 6. The reverse of dehydration...Ch. 2 - Fill in the Blanks 7. Reactions that release...Ch. 2 - Fill in the Blanks 8. All chemical reactions begin...Ch. 2 - Fill in the Blanks 9. The ____________ scale is a...Ch. 2 - Prob. 10FIBCh. 2 - Label a portion of the molecule below; label two...Ch. 2 - Shown is the amino acid tryptophan. Put the letter...Ch. 2 - List three main types of chemical bonds, and give...Ch. 2 - Name five properties of water that are vital to...Ch. 2 - Prob. 3SACh. 2 - What is the difference between atomic oxygen and...Ch. 2 - Explain how the polarity of water molecules makes...Ch. 2 - Prob. 1CTCh. 2 - Prob. 2CTCh. 2 - Two freshmen disagree about an aspect of...Ch. 2 - When an egg white is heated, it changes from...Ch. 2 - Prob. 5CTCh. 2 - The poison glands of many bees and wasps contain...Ch. 2 - Prob. 7CTCh. 2 - Prob. 8CTCh. 2 - The deadly poison hydrogen cyanide has the...Ch. 2 - Triple covalent bonds are stronger and more...Ch. 2 - How can hydrogen bonding between water molecules...Ch. 2 - How can a single molecule of magnesium hydroxide...Ch. 2 - Prob. 13CTCh. 2 - Prob. 14CTCh. 2 - A textbook states that only five nucleotide bases...Ch. 2 - Using the following terms, fill in the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- This question has multiple parts (A, B & C), and under the subject of Nutrition. Thank you!arrow_forwardCalculate the CFU/ml of a urine sample if 138 E. coli colonies were counted on a Nutrient Agar Plate when0.5 mls were plated on the NA plate from a 10-9 dilution tube. You must highlight and express your answerin scientific notatioarrow_forwardDon't copy off the other answer if there is anyarrow_forward
- Use the following information to answer the question(s) below. Martin Wikelski and L. Michael Romero (Body size, performance and fitness in Galápagos marine iguanas, Integrative and Comparative Biology 43 [2003]:376-86) measured the snout-to-vent (anus) length of Galápagos marine iguanas and observed the percent survival of different-sized animals, all of the same age. The graph shows the log snout-vent length (SVL, a measure of overall body size) plotted against the percent survival of these different size classes for males and females. Survival (%) 100- 80- 60- 40- 20- 0+ 1.9 T 2 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Log SVL (mm) 19) Examine the figure above. What type of selection for body size appears to be occurring in these marine iguanas? A) directional selection B) stabilizing selection C) disruptive selection D) You cannot determine the type of selection from the above information. 3arrow_forward24) Use the following information to answer the question below. Researchers studying a small milkweed population note that some plants produce a toxin and other plants do not. They identify the gene responsible for toxin production. The dominant allele (T) codes for an enzyme that makes the toxin, and the recessive allele (t) codes for a nonfunctional enzyme that cannot produce the toxin. Heterozygotes produce an intermediate amount of toxin. The genotypes of all individuals in the population are determined (see table) and used to determine the actual allele frequencies in the population. TT 0.49 Tt 0.42 tt 0.09 Refer to the table above. Is this population in Hardy-Weinberg equilibrium? A) Yes. C) No; there are more homozygotes than expected. B) No; there are more heterozygotes than expected. D) It is impossible to tell.arrow_forward30) A B CDEFG Refer to the accompanying figure. Which of the following forms a monophyletic group? A) A, B, C, and D B) C and D C) D, E, and F D) E, F, and Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
 Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning


Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning

Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license