
Concept explainers
Mercury Emissions by Continent By weight, coal does not contain much mercury, but we burn a lot of it. Several industries besides coal-fired power plants contribute substantially to atmospheric mercury pollution. FIGURE 2.13 shows mercury emissions by industry from different regions of the world in 2010.
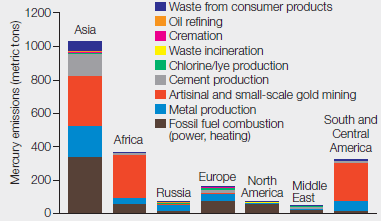
FIGURE 2.13 Global mercury emissions by sector, 2010.
About how many metric tons of mercury were released in total from these regions?
To determine: The total metric tons of mercury released from the regions given in the Fig. 2.13.
Introduction: Mercury is a toxic element and is naturally found in water, air, and soil. It is released in the environment through weathering of rocks, volcanic activity, and human activity. Human activity is mainly responsible for the mercury releases especially burning of coal in the coal-fired power stations, domestic uses, waste incinerators, industries, and mining of metals.
Explanation of Solution
As given in the problem statement, various industries besides coal-fired power plants contribute to mercury pollution in the atmosphere. Refer Fig. 2.13, “Global mercury emissions by sector, 2010” in the textbook. The graphical representation shows mercury emission (metric tons) by industries from different continents of the world in 2010. The data were reported from seven continents including Asia, Africa, Russia, Europe, North America, Middle East, and South and Central America. The mercury emissions released from various sectors included waste from consumer products, oil refining, cremation, waste incineration, mining, fossil fuel combustion, chlorine, cement, and metal production. On the basis of the data given in Fig. 2.13, Asia has the maximum mercury emissions reported as approximately 1100 metric tons. The approximate amount of mercury released by each continent is as follows:
- Asia – 1100 metric tons
- Africa – 380 metric tons
- South and Central America – 350 metric tons
- Europe – 180 metric tons
- North America – 80 metric tons
- Russia – 60 metric tons
- Middle East – 40 metric tons
By summing up the amount of mercury emissions from the seven continents, the total amount of mercury released (metric tons) comes to approximately 2200 metric tons.
The total mercury released by industry from different regions of the world in 2010 was 2200 metric tons.
Want to see more full solutions like this?
Chapter 2 Solutions
Biology: The Unity and Diversity of Life (Looseleaf)
Additional Science Textbook Solutions
Biological Science (6th Edition)
Physical Science
Genetics: From Genes to Genomes
Campbell Essential Biology (7th Edition)
Microbiology Fundamentals: A Clinical Approach
- the direct output from molecular replacement is a coordinate file showing the orientation of the unknown target protein in the unit cell. true or false?arrow_forwardDid your intake of vitamin C meet or come very close to the recommended amount? yes noarrow_forwardWhich of the following statements about hydration is true? Absence of thirst is a reliable indication that an individual is adequately hydrated. All of these statements are true. Although a popular way to monitor hydration status, weighing yourself before and after intensive physical activity is not a reliable method to monitor hydration. Urine that is the color of apple juice indicates dehydration. I don't know yetarrow_forward
- Three of the many recessive mutations in Drosophila melanogaster that affect body color, wing shape, or bristle morphology are black (b) body versus grey in wild type, dumpy (dp), obliquely truncated wings versus long wings in the male, and hooked (hk) bristles versus not hooked in the wild type. From a cross of a dumpy female with a black and hooked male, all of the F1 were wild type for all three of the characters. The testcross of an F1 female with a dumpy, black, hooked male gave the following results: Trait Number of individuals Wild type 169 Black 19 Black, hooked 301 Dumpy, hooked 21 Hooked, dumpy, black 172 Dumpy, black 6 Dumpy 305 Hooked 8 Determine the order of the genes and the mapping distance between genes. Determine the coefficient of confidence for the portion of the chromosome involved in the cross. How much interference takes place in the cross?arrow_forwardWhat happens to a microbes membrane at colder temperature?arrow_forwardGenes at loci f, m, and w are linked, but their order is unknown. The F1 heterozygotes from a cross of FFMMWW x ffmmww are test crossed. The most frequent phenotypes in the test cross progeny will be FMW and fmw regardless of what the gene order turns out to be. What classes of testcross progeny (phenotypes) would be least frequent if locus m is in the middle? What classes would be least frequent if locus f is in the middle? What classes would be least frequent if locus w is in the middle?arrow_forward
- 1. In the following illustration of a phospholipid... (Chemistry Primer and Video 2-2, 2-3 and 2-5) a. Label which chains contain saturated fatty acids and non-saturated fatty acids. b. Label all the areas where the following bonds could form with other molecules which are not shown. i. Hydrogen bonds ii. Ionic Bonds iii. Hydrophobic Interactions 12-6 HICIH HICIH HICHH HICHH HICIH OHHHHHHHHHHHHHHHHH C-C-C-C-C-c-c-c-c-c-c-c-c-c-c-c-C-C-H HH H H H H H H H H H H H H H H H H H HO H-C-O H-C-O- O O-P-O-C-H H T HICIH HICIH HICIH HICIH HHHHHHH HICIH HICIH HICIH 0=C HIC -C-C-C-C-C-C-C-C-CC-C-C-C-C-C-C-C-C-H HHHHHHHHH IIIIIIII HHHHHHHH (e-osbiv)arrow_forwardAnswer this as a dental assistant studentarrow_forwardbuatkan judul skripsi tentang parasitologi yang sedang trendinharrow_forward
- Dental assistantarrow_forwardO Macmillan Learning Glu-His-Trp-Ser-Gly-Leu-Arg-Pro-Gly The pKa values for the peptide's side chains, terminal amino groups, and carboxyl groups are provided in the table. Amino acid Amino pKa Carboxyl pKa Side-chain pKa glutamate 9.60 2.34 histidine 9.17 1.82 4.25 6.00 tryptophan 9.39 2.38 serine 9.15 2.21 glycine 9.60 2.34 leucine 9.60 2.36 arginine 9.04 2.17 12.48 proline 10.96 1.99 Calculate the net charge of the molecule at pH 3. net charge at pH 3: Calculate the net charge of the molecule at pH 8. net charge at pH 8: Calculate the net charge of the molecule at pH 11. net charge at pH 11: Estimate the isoelectric point (pl) for this peptide. pl:arrow_forwardBiology Questionarrow_forward
 Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning





