
Inorganic Chemistry
5th Edition
ISBN: 9781292134147
Author: Housecroft, Catherine E.
Publisher: Pearson,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 18P
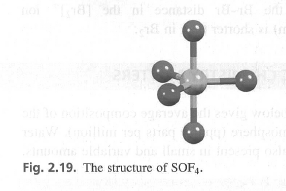
Use the VSEPR model to rationalize the structure of
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
b.
CH3
H3C
CH3
CH3
H3C
an unexpected product,
containing a single 9-
membered ring
the expected product,
containing two fused rings
H3C-I
(H3C)2CuLi
an enolate
b.
H3C
CH3
1.
2. H3O+
H3C
MgBr
H3C
Predict the major products of this reaction:
excess
H+
NaOH
?
A
Note that the first reactant is used in excess, that is, there is much more of the first reactant than the second.
If there won't be any products, just check the box under the drawing area instead.
Explanation
Check
Click and drag to start drawing a
structure.
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Priv
Chapter 2 Solutions
Inorganic Chemistry
Ch. 2 - Draw Lewis structures to describe the bonding in...Ch. 2 - Use the Lewis structure model to deduce the type...Ch. 2 - Draw the resonance structures for the O3 molecule....Ch. 2 - 2.4 Draw Lewis structures for (a) , (b) ,(c) and...Ch. 2 - 2.5 Each of the following is a radical. For which...Ch. 2 - (a) Use VB theory to describe the bonding in the...Ch. 2 - 2.7 Use VB theory and Lewis structure model,...Ch. 2 - 2.8 Does VB theory indicate that the diatomic...Ch. 2 - 2.9 (a) Use MO theory to determine the bond order...Ch. 2 - Prob. 10P
Ch. 2 - Prob. 11PCh. 2 - Draw charge-separated resonance structures to give...Ch. 2 - Prob. 13PCh. 2 - In the following table, match a species in list 1...Ch. 2 - Using the data in table 2.2, determine which of...Ch. 2 - Prob. 16PCh. 2 - 2.17 Use the VSEPR model to predict the structures...Ch. 2 - 2.18 Use the VSEPR model to rationalize the...Ch. 2 - Determine the shapes of each of the following...Ch. 2 - 2.20 State whether you expect the following...Ch. 2 - 2.21 (a) Draw resonance structure for the CO,...Ch. 2 - Prob. 22PCh. 2 - Prob. 23PCh. 2 - Suggest reasons for the following observations....Ch. 2 - Prob. 25PCh. 2 - Prob. 26PCh. 2 - 2.27 (a) Write down the ions that are present in...Ch. 2 - 2.28 Assuming that VSEPR model can be applied...Ch. 2 - Critically compare the VB and MO treatments of the...Ch. 2 - The table below gives the average composition of...Ch. 2 - Carbon monoxide is a toxic pollutant which arises...Ch. 2 - 2.32 Volcanoes and deep sea hydrothermal vents are...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Give the IUPAC name for each compound.
Organic Chemistry
1. Genetics affects many aspects of our lives. Identify three ways genetics affects your life or the life of a ...
Genetic Analysis: An Integrated Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as indicated by the boxes. a. NaOMe H+ .CO,H HO₂C MeOH (excess) MeOH H3C Br يع CH3 1. LiAlH4 2. H3O+ 3. PBг3 H3C 1. Et-Li 2. H3O+ -CO₂Me -CO₂Me OH CH3 CH3 ল CH3arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe ག1, ད།་, - + H You can draw 1 and 2 in any arrangement you like. 2 work up Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Parrow_forwardWhat is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forward
- ΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forward
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY