
Chemistry in Context
8th Edition
ISBN: 9780073522975
Author: American Chemical Society
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 16Q
Consider these two waves representing different parts of the
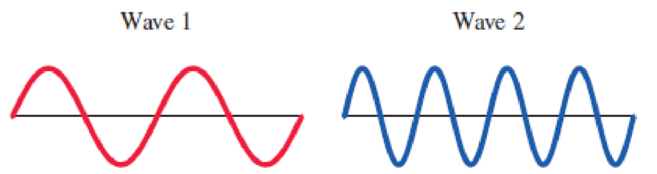
- a. wavelength
- b. frequency
- c. speed of travel
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
13
How many signals would you expect to see in the
Check
O
signal(s)
X
§
'C NMR spectrum for the following compound?
© 2025 McGraw Hill
13
Consider the "C NMR spectrum below.
140
120
100
80
60
40
20
20
PPM
0
The spectrum belongs to which one of the following constitutional isomers of the compound C,H12? Select the single best answer.
Check
✓
G
Save For Later
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use
The structure of compound 1,3,5-trimethylbenzene (mesitylene) is given below.
How many signals would you expect to find in the 'H NMR spectrum of 1,3,5-trimethylbenzene (mesitylene)?
Check
×
Chapter 2 Solutions
Chemistry in Context
Ch. 2.1 - Prob. 2.3CTCh. 2.6 - Prob. 2.15CTCh. 2.7 - Prob. 2.17CTCh. 2.7 - Prob. 2.19SCCh. 2.7 - The indoor tanning industry runs a public...Ch. 2.7 - Prob. 2.21CTCh. 2.8 - Prob. 2.22CTCh. 2.8 - Prob. 2.23YTCh. 2.11 - Prob. 2.27CTCh. 2.13 - Prob. 2.30CT
Ch. 2.13 - Prob. 2.32CTCh. 2 - How does ozone differ from oxygen in its chemical...Ch. 2 - Prob. 2QCh. 2 - Prob. 3QCh. 2 - Prob. 4QCh. 2 - Prob. 5QCh. 2 - Prob. 6QCh. 2 - a. What is a Dobson unit? b. Does a reading of 320...Ch. 2 - Using the periodic table as a guide, specify the...Ch. 2 - Consider this representation of a periodic table....Ch. 2 - Give the name and symbol for the element with this...Ch. 2 - Give the number of protons, neutrons, and...Ch. 2 - Give the symbol showing the atomic number and the...Ch. 2 - Prob. 13QCh. 2 - Assuming that the octet rule applies, draw the...Ch. 2 - Prob. 15QCh. 2 - Consider these two waves representing different...Ch. 2 - Prob. 17QCh. 2 - Prob. 18QCh. 2 - Arrange these types of radiation in order of...Ch. 2 - The microwaves in home microwave ovens have a...Ch. 2 - Ultraviolet radiation is categorized as UVA, UVB,...Ch. 2 - Prob. 22QCh. 2 - Prob. 23QCh. 2 - Prob. 24QCh. 2 - Prob. 25QCh. 2 - The following free radicals all play a role in...Ch. 2 - a. How were the original measurements of increases...Ch. 2 - Prob. 28QCh. 2 - The EPA has used the slogan Ozone: Good Up High,...Ch. 2 - Nobel Laureate F. Sherwood Rowland referred to the...Ch. 2 - Prob. 31QCh. 2 - Prob. 32QCh. 2 - Prob. 33QCh. 2 - Prob. 34QCh. 2 - Prob. 35QCh. 2 - Prob. 36QCh. 2 - The average length of an OO single bond is 132 pm....Ch. 2 - Prob. 38QCh. 2 - Prob. 39QCh. 2 - Prob. 40QCh. 2 - All the reports of the damage caused by UV...Ch. 2 - Prob. 42QCh. 2 - Prob. 43QCh. 2 - Prob. 44QCh. 2 - Development of the stratospheric ozone hole has...Ch. 2 - Prob. 46QCh. 2 - One mechanism that helps break down ozone in the...Ch. 2 - Polar stratospheric clouds (PSCs) play an...Ch. 2 - Prob. 49QCh. 2 - Prob. 50QCh. 2 - Resonance structures can be used to explain the...Ch. 2 - Prob. 52QCh. 2 - Prob. 53QCh. 2 - Many different types of ozone generators...Ch. 2 - The effect a chemical substance has on the ozone...Ch. 2 - Prob. 56Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1 How many signals do you expect in the 'H NMR spectrum for this molecule? CI CI Cl Write the answer in the table below. Also, in each of the drawing areas below is a copy of the molecule, with H atoms shown. In each copy, one of the H atoms is highlighted red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: Remember, a multiplet is considered one signal in the 'H NMR spectrum. 1 Number of signals in the 'H NMR spectrum. ☐ For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional H atoms to highlight in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at…arrow_forwardwrtie the balanced equation and find the E° when the following half- reactions are combined Zn2+(aq) + 2e---> Zn(s) E°= -0.763V Ag+(aq) + e---> Ag (s) E°=+0.799Varrow_forwardConsider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 'H NMR spectrum? Note: A multiplet is considered one signal. ☐arrow_forward
- Study this 'H NMR spectrum, and then answer the questions about it in the table below. Check 1.0- 0.5- 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 What unit symbol should be written on the horizontal axis? What is the chemical shift & of the doublet? If there is no doublet, just check the box instead. Give your answer to 2 significant digits. What is the chemical shift of the signal immediately upfield of the doublet? If there is no doublet, or no signal upfield of it, check the box instead. What is the chemical shift & of the least deshielded proton? If you can't tell without more information, check the box instead. 血 8 = ☐ There is no doublet. 8 = ☐ No such signal. 8 = 0 Need more information.arrow_forwardhow many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20arrow_forwardcalculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2arrow_forward
- an adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank providearrow_forwardWhat are the advantages and/or disadvantages of using the MOHR titration method & AOEC method?arrow_forwardAre there any alternative methods better than the MOHR titration to quantitatively determine salt in a sample?arrow_forward
- hybridization of nitrogen of complex moleculesarrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO2 (g) = N2O4(g) AGº = -5.4 kJ Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system: Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to '2' rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. 00 rise ☐ x10 fall yes no ☐ atm G Ar 1arrow_forwardWhy do we analyse salt?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Quantum Mechanics - Part 1: Crash Course Physics #43; Author: CrashCourse;https://www.youtube.com/watch?v=7kb1VT0J3DE;License: Standard YouTube License, CC-BY