
BIOCHEMISTRY BOOKS ALC&MOD MST/ET PKG
1st Edition
ISBN: 9780134172507
Author: APPLING
Publisher: Pearson Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 15P
The anno acid arginine ionizes according to the following scheme:
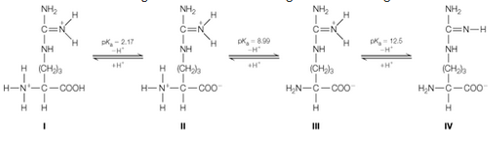
a. Calculate the isoelectric point of arginine. You can neglect contributions from form l. Why?
b. Calculate the average charge on arginine when pH = 9.20. (Hint: Find the average charge for each ionizable group and sum these together)
c. Is the value of average charge you calculated in part b reasonable, given the pl you calculated part a? Explain your answer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show work. don't give Ai generated solution....give correct solution
Biochemistry
What is the process of "transamination" in either the muscles or the liver, that involves keto acid or glutamic acid?
Please explain how the steps work. Thank you!
Biochemistry
Please help. Thank you
What is the importance of glutamic acid in the metabolism of nitrogen from amino acids? (we know therole; it’s used to remove the nitrogen from amino acids so that the remaining carbon skeleton can bebroken down by the “usual” pathways, but what is the important, unique role that only glutamicacid/glutamate can do?)
Chapter 2 Solutions
BIOCHEMISTRY BOOKS ALC&MOD MST/ET PKG
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - Prob. 20PCh. 2 - Prob. 21P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Biochemistry Please help. Thank you When carbamyl phosphate is joined to L-ornathine, where does the energy for the reaction come from?arrow_forwardBiochemistry Question Please help. Thank you What is the function of glutamate dehydrogenase?arrow_forwardBiochemistry Question Please help. Thank you How and why does a high protein diet affect the enzymes of the urea cycle?arrow_forward
- Biochemistry What is the importance of the glucose-alanine cycle?arrow_forwardBiochemistry Assuming 2.5 molecules of ATP per oxidation of NADH/(H+) and 1.5molecules of ATP per oxidation of FADH2, how many ATP are produced per molecule of pyruvate? Please help. Thank youarrow_forward1. How would you explain the term ‘good food’? 2. How would you define Nutrition? 3. Nutrients are generally categorised into two forms. Discuss.arrow_forward
- Biochemistry Question. Please help solve. Thank you! Based upon knowledge of oxidation of bioorganic compounds and howmuch energy is released during their oxidation, rank the following, from most to least, with respect to how much energy would be produced from each during their oxidation. Explain your placement for each one.arrow_forwardBiochemistry Question.For the metabolism of amino acids what is the first step for theirbreakdown? Why is it necessary for this breakdown product to be transported to the liver? For the catabolism of the carbon backbone of these amino acids, there are 7 entry points into the “standard” metabolic pathways. List these 7 entry points and which amino acids are metabolized to these entry points. Please help. Thank you!arrow_forwardBiochemistry Question. Please help. Thank you. You are studying pyruvate utilization in mammals for ATP production under aerobic conditions and have synthesized pyruvate with Carbon #1 labelled with radioactive C14. After only one complete cycle of the TCA cycle, which of the TCA cycle intermediates would be labeled with C14? Explain your answer. Interestingly, you find C14 being excreted in the urine. How does it get there?arrow_forward
- Biochemistry question. Please help with. Thanks in advance For each of the enzymes listed below, explain what the enzyme does including function, names (or structures) of the substrate and products and the pathway(s) (if applicable) it is/are found in. (a) ATP synthetase (b) succinate dehydrogenase (c) isocitrate lyase (d) acetyl CoA carboxylase (e) isocitrate dehydrogenase (f) malate dehydrogenasearrow_forwardDraw and name each alcohol and classify it as primary, secondary, or tertiary. Explain your answer thoroughly.arrow_forwardDraw the product of each reaction. If there are multiple products, draw only the major product. Explain your answer thoroughly.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning



Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license