
Concept explainers
Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
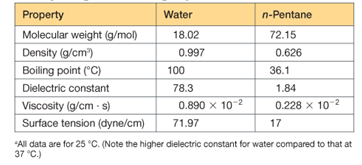
the interaction energy (the energy required to pull them infinitely far apart) predicted to be larger if the medium between them is water, or if it is n-pentane? (See Table 2.5)
If Ca2+, Na+ and F¯ each have ionic radii ~1.16. Which ionic bond is stronger: Ca-F or Na-F? If Ca2+ is often bound on the surface of a protein by
Table 2.5 Important properties of liquid water compared with those of n-pentane, a nonpolar,
Nonhydrogen-bonding liquida
Interpretation:
Interaction energy will be more in which of the given ion pairs must be predicted. The stronger ionic bond must be predicted among the given ionic compounds. In which of the given pH the interaction between
Concept introduction:
Ionic interaction depends on Columbic interaction which depends on the charge of ions and distance by which those ions are separated. It aslo depends on the dielectric constant of the medium and the viscosity of the medium. The ionic bond is stronger when the size of the ions is small, charge in ions is high. Lattice energy depends on Columbic interaction. The bond between
Answer to Problem 1P
The interaction energy between sodium ion and chlorine ion will be larger in case of n-pentane.
Explanation of Solution
The interaction of ion pair will be more in case of n-pentane.
This is because the dielectric constant of n-pentane is
Now,
This is because of Columbic interaction
where
As calcium ion has the double charge of sodium ion so
The
This is because at pH greater than
So, the best pH is
Want to see more full solutions like this?
Chapter 2 Solutions
BIOCHEMISTRY BOOKS ALC&MOD MST/ET PKG
Additional Science Textbook Solutions
Campbell Essential Biology with Physiology (5th Edition)
Microbiology with Diseases by Body System (5th Edition)
Anatomy & Physiology (6th Edition)
Biology: Life on Earth with Physiology (11th Edition)
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
- Br Mg, ether 1. HCHO (formaldehyde) 2. H+, H₂O PCC 1. NH3, HCN ? (pyridinium chlorochromate) 2. H2O, HCI 11. Which one of the following compounds is the major organic product of the series of reactions shown above? Ph. Ph. OH NH2₂ A Ph. Ή NH2 B OH Ph Η Ph OH NH2 NH2₂ NH₂ C D Earrow_forwardB A 6. Which ONE of the labeled bonds in the tripeptide on the right is a peptide bond: H₂N N 'N' OH C H A, B, C, D or E? HN E OHarrow_forwardQuestions 8-9 are 0.4 points each. The next two questions relate to the peptide whose structure is shown here. To answer these questions, you should look at a table of H2N/.. amino acid structures. You don't have to memorize the structures of the amino acids. IZ 8. What is the N-terminal amino acid of this peptide? A) proline B) aspartic acid C) threonine 9. What is the C-terminal amino acid of this peptide? A) proline B) aspartic acid C) threonine N OH D) valine E) leucine D) valine E) leucine NH "OH OHarrow_forward
- 7. What is the correct name of the following tripeptide? A) Ile-Met-Ser B) Leu-Cys-Thr C) Val-Cys-Ser D) Ser-Cys-Leu E) Leu-Cys-Ser H₂N!!!!! N H ΖΙ .SH SF H IN OH OHarrow_forwardPlease draw out the following metabolic pathways: (Metabolic Map) Mitochondrion: TCA Cycle & GNG, Electron Transport, ATP Synthase, Lipolysis, Shuttle Systems Cytoplasm: Glycolysis & GNG, PPP (Pentose Phosphate Pathway), Glycogen, Lipogenesis, Transporters and Amino Acids Control: Cori/ Glc-Ala cycles, Insulin/Glucagon Reg, Local/Long Distance Regulation, Pools Used Correctlyarrow_forwardPlease help provide me an insight of what to draw for the following metabolic pathways: (Metabolic Map) Mitochondrion: TCA Cycle & GNG, Electron Transport, ATP Synthase, Lipolysis, Shuttle Systems Cytoplasm: Glycolysis & GNG, PPP (Pentose Phosphate Pathway), Glycogen, Lipogenesis, Transporters and Amino Acids Control: Cori/ Glc-Ala cycles, Insulin/Glucagon Reg, Local/Long Distance Regulation, Pools Used Correctlyarrow_forward
- f. The genetic code is given below, along with a short strand of template DNA. Write the protein segment that would form from this DNA. 5'-A-T-G-G-C-T-A-G-G-T-A-A-C-C-T-G-C-A-T-T-A-G-3' Table 4.5 The genetic code First Position Second Position (5' end) U C A G Third Position (3' end) Phe Ser Tyr Cys U Phe Ser Tyr Cys Leu Ser Stop Stop Leu Ser Stop Trp UCAG Leu Pro His Arg His Arg C Leu Pro Gln Arg Pro Leu Gin Arg Pro Leu Ser Asn Thr lle Ser Asn Thr lle Arg A Thr Lys UCAG UCAC G lle Arg Thr Lys Met Gly Asp Ala Val Gly Asp Ala Val Gly G Glu Ala UCAC Val Gly Glu Ala Val Note: This table identifies the amino acid encoded by each triplet. For example, the codon 5'-AUG-3' on mRNA specifies methionine, whereas CAU specifies histidine. UAA, UAG, and UGA are termination signals. AUG is part of the initiation signal, in addition to coding for internal methionine residues. Table 4.5 Biochemistry, Seventh Edition 2012 W. H. Freeman and Company B eviation: does it play abbreviation:arrow_forwardAnswer all of the questions please draw structures for major productarrow_forwardfor glycolysis and the citric acid cycle below, show where ATP, NADH and FADH are used or formed. Show on the diagram the points where at least three other metabolic pathways intersect with these two.arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College





