
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 132GQ
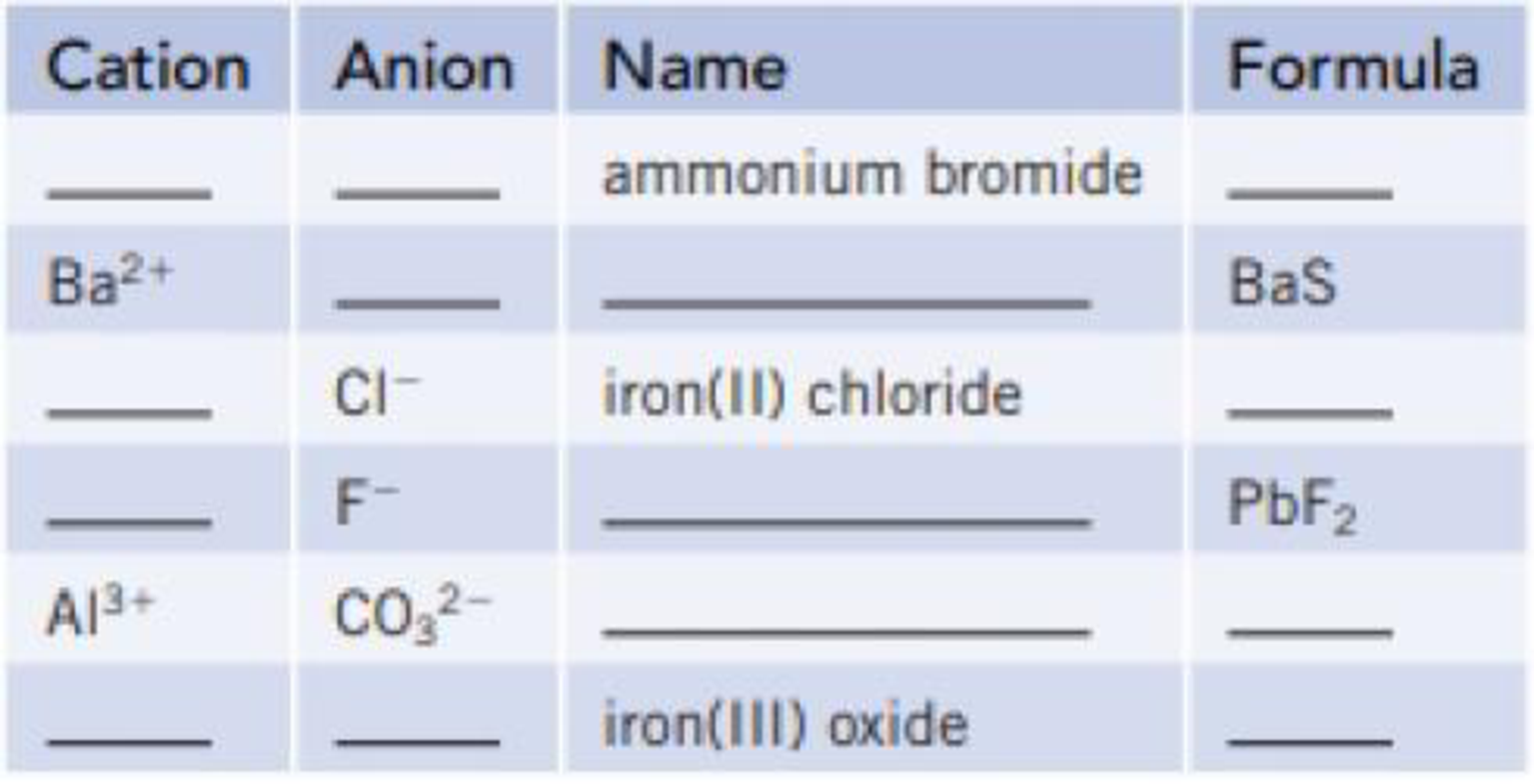
Complete the table by placing symbols, formulas, and names in the blanks.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
3.Help
4. Help
10. Help
Chapter 2 Solutions
Chemistry & Chemical Reactivity
Ch. 2.1 - 1. What is the mass of an iron atom with 30...Ch. 2.2 - Verify that the atomic weight of chlorine is...Ch. 2.2 - Neon has three stable isotopes, one with a small...Ch. 2.5 - Give the number and identity of the constituent...Ch. 2.5 - (a) Write the formulas of all neutral ionic...Ch. 2.6 - What mass of gold, Au, contains 2.6 1024 atoms?Ch. 2.6 - If you have 454 g of citric acid (H3C6H5O7), what...Ch. 2.7 - 1. Express the composition of ammonium carbonate,...Ch. 2.7 - 1. What is the empirical formula of naphthalene,...Ch. 2.7 - Gallium oxide, GaxOy forms when gallium is combine...
Ch. 2.7 - Hydrated nickel(II) chloride is a beautiful green,...Ch. 2.8 - Prob. 2.12CYUCh. 2.8 - Prob. 1.1ACPCh. 2.8 - Prob. 1.2ACPCh. 2.8 - Prob. 2.1ACPCh. 2.8 - Salvarsan was long thought to be a single...Ch. 2.8 - To determine the density of atmospheric nitrogen....Ch. 2.8 - The density of a mixture of gases may be...Ch. 2.8 - Atmospheric argon is a mixture of three stable...Ch. 2.8 - Given that the density of argon is 1.78 g/L under...Ch. 2 - Prob. 1PSCh. 2 - Define mass number. What is the difference between...Ch. 2 - An atom has a very small nucleus surrounded by an...Ch. 2 - A gold atom has a radius of 145 pm. If you could...Ch. 2 - Give the complete symbol(ZAX), including atomic...Ch. 2 - Give the complete symbol(ZAX), including atomic...Ch. 2 - Prob. 7PSCh. 2 - Atomic structure. (a) The synthetic radioactive...Ch. 2 - Prob. 9PSCh. 2 - In 1886 Eugene Goldstein observed positively...Ch. 2 - Marie Curie was born in Poland but studied and...Ch. 2 - Prob. 12PSCh. 2 - The mass of an 16 O atom is 15.995 u. What is its...Ch. 2 - What is the mass of one 16O atom, in grams? (The...Ch. 2 - Cobalt has three radioactive isotopes used in...Ch. 2 - Naturally occurring silver exists as two isotopes...Ch. 2 - Name and describe the composition of the three...Ch. 2 - Which of the following are isotopes of element X,...Ch. 2 - Thallium has two stable isotopes, 203TIand 205Tl....Ch. 2 - Strontium has four stable isotopes. Strontium-84...Ch. 2 - Verify that the atomic weight of lithium is 6.94,...Ch. 2 - Verify that the atomic weight of magnesium is...Ch. 2 - Gallium has two naturally occurring isotopes, 69Ga...Ch. 2 - Europium has two stable isotopes, 151Eu and 153Eu,...Ch. 2 - Titanium and thallium have symbols that are easily...Ch. 2 - In Groups 4A-6A, there are several elements whose...Ch. 2 - How many periods of the periodic table have 8...Ch. 2 - Prob. 28PSCh. 2 - Prob. 29PSCh. 2 - Prob. 30PSCh. 2 - Classify the following elements as metals,...Ch. 2 - Prob. 32PSCh. 2 - Prob. 33PSCh. 2 - Prob. 34PSCh. 2 - What is the charge on the common monatomic ions of...Ch. 2 - What is the charge on the common monatomic ions of...Ch. 2 - Prob. 37PSCh. 2 - Prob. 38PSCh. 2 - When a potassium atom becomes a monatomic ion, how...Ch. 2 - When oxygen and sulfur atoms become monatomic...Ch. 2 - Prob. 41PSCh. 2 - Prob. 42PSCh. 2 - Give the formula and the number of each ion that...Ch. 2 - Give the formula and the number of each ion that...Ch. 2 - Prob. 45PSCh. 2 - Prob. 46PSCh. 2 - Prob. 47PSCh. 2 - Prob. 48PSCh. 2 - Prob. 49PSCh. 2 - Prob. 50PSCh. 2 - Prob. 51PSCh. 2 - Prob. 52PSCh. 2 - Write the formulas for the four ionic compounds...Ch. 2 - Write the formulas for the four ionic compounds...Ch. 2 - Sodium ions, Na+, form ionic compounds with...Ch. 2 - Consider the two ionic compounds NaCl and CaO. In...Ch. 2 - Prob. 57PSCh. 2 - Name each of the following binary, nonionic...Ch. 2 - Prob. 59PSCh. 2 - Prob. 60PSCh. 2 - Calculate the mass, in grams, of each the...Ch. 2 - Calculate the mass, in grams, of each the...Ch. 2 - Calculate the amount (moles) represented by each...Ch. 2 - Calculate the amount (moles) represented by each...Ch. 2 - You are given 1.0-g samples of He, Fe, Li, Si, and...Ch. 2 - You are given 0.10-g samples of K, Mo, Cr, and Al....Ch. 2 - Analysis of a 10.0-g sample of apatite (a major...Ch. 2 - A semiconducting material is composed of 52 g of...Ch. 2 - Calculate the molar mass of each of the following...Ch. 2 - Calculate the molar mass of each of the following...Ch. 2 - Calculate the molar mass of each hydrated...Ch. 2 - Prob. 72PSCh. 2 - What mass is represented by 0.0255 mol of each of...Ch. 2 - Assume you have 0.123 mol of each of the following...Ch. 2 - Sulfur trioxide, SO3, is made industrially in...Ch. 2 - How many ammonium ions and how many sulfate ions...Ch. 2 - Acetaminophen, whose structure is drawn below, is...Ch. 2 - An Alka-Seltzer tablet contains 324 mg of aspirin...Ch. 2 - Calculate the mass percent of each element in the...Ch. 2 - Calculate the mass percent of each element in the...Ch. 2 - Calculate the mass percent of copper in CuS,...Ch. 2 - Calculate the mass percent of titanium in the...Ch. 2 - Succinic acid occurs in fungi and lichens. Its...Ch. 2 - An organic compound has the empirical formula...Ch. 2 - Prob. 85PSCh. 2 - Complete the following table:Ch. 2 - Acetylene is a colorless gas used as a fuel in...Ch. 2 - A large family of boron-hydrogen compounds has the...Ch. 2 - Cumene, a hydrocarbon, is a compound composed only...Ch. 2 - In 2006, a Russian team discovered an interesting...Ch. 2 - Mandelic acid is an organic acid composed of...Ch. 2 - Nicotine, a poisonous compound found in tobacco...Ch. 2 - A compound containing xenon and fluorine was...Ch. 2 - Elemental sulfur (1.256 g) is combined with...Ch. 2 - Epsom salt is used in tanning leather and in...Ch. 2 - You combine 1.25 g of germanium, Ge, with excess...Ch. 2 - The mass spectrum of nitrogen dioxide is...Ch. 2 - The mass spectrum of phosphoryl chloride. POF3, is...Ch. 2 - The mass spectrum of CH3Cl is illustrated here....Ch. 2 - Prob. 100PSCh. 2 - Fill in the blanks in the table (one column per...Ch. 2 - Potassium has three naturally occurring isotopes...Ch. 2 - Crossword Puzzle: In the 2 2 box shown here, each...Ch. 2 - The following chart shows a general decline in...Ch. 2 - Copper atoms. (a) What is the average mass of one...Ch. 2 - Prob. 106GQCh. 2 - Prob. 107GQCh. 2 - Identify two nonmetallic elements that have...Ch. 2 - Prob. 109GQCh. 2 - Prob. 110GQCh. 2 - Prob. 111GQCh. 2 - When a sample of phosphorus burns in air, the...Ch. 2 - Although carbon-12 is now used as the standard for...Ch. 2 - A reagent occasionally used in chemical synthesis...Ch. 2 - Prob. 115GQCh. 2 - Prob. 116GQCh. 2 - Which of the following compounds has the highest...Ch. 2 - Which of the following samples has the largest...Ch. 2 - The structure of one of the bases in DNA, adenine,...Ch. 2 - Prob. 120GQCh. 2 - A drop of water has a volume of about 0.050 mL....Ch. 2 - Capsaicin, the compound that gives the hot taste...Ch. 2 - Prob. 123GQCh. 2 - Write the molecular formula and calculate the...Ch. 2 - Malic acid, an organic acid found in apples,...Ch. 2 - Your doctor has diagnosed you as being anemicthat...Ch. 2 - A compound composed of iron and carbon monoxide,...Ch. 2 - Ma huang, an extract from the ephedra species of...Ch. 2 - Saccharin, a molecular model of which is shown...Ch. 2 - Prob. 130GQCh. 2 - Write the formula for each of the following pounds...Ch. 2 - Complete the table by placing symbols, formulas,...Ch. 2 - Empirical and molecular formulas. (a)...Ch. 2 - Cacodyl, a compound containing arsenic, was...Ch. 2 - The action of bacteria on meat and fish produces a...Ch. 2 - In the laboratory you combine 0.125 g of nickel...Ch. 2 - A compound called MMT was once used to boost the...Ch. 2 - Elemental phosphorus is made by heating calcium...Ch. 2 - Chromium is obtained by heating chromium(III)...Ch. 2 - Stibnite, Sb2S3, is a dark gray mineral from which...Ch. 2 - Direct reaction of iodine (I2) and chlorine (Cl2)...Ch. 2 - In a reaction, 2.04 g of vanadium combined with...Ch. 2 - Iron pyrite, often called fools gold, has the...Ch. 2 - Which of the following statements about 57.1 g of...Ch. 2 - The formula of barium molybdate is BaMoO4. Which...Ch. 2 - A metal M forms a compound with the formula MCl4....Ch. 2 - Pepto-Bismol, which can help provide relief for an...Ch. 2 - The weight percent of oxygen in an oxide that has...Ch. 2 - The mass of 2.50 mol of a compound with the...Ch. 2 - The elements A and Z combine to produce two...Ch. 2 - Polystyrene can be prepared by heating styrene...Ch. 2 - A sample of hemoglobin is found to be 0.335% iron....Ch. 2 - Consider an atom of 64Zn. (a) Calculate the...Ch. 2 - Estimating the radius of a lead atom. (a) You are...Ch. 2 - A piece of nickel foil, 0.550 mm thick and 1.25 cm...Ch. 2 - Uranium is used as a fuel, primarily in the form...Ch. 2 - In an experiment, you need 0.125 mol of sodium...Ch. 2 - Mass spectrometric analysis showed that there are...Ch. 2 - If Epsom salt, MgSO4 x H2O, is heated to 250 C,...Ch. 2 - The alum used in cooking is potassium aluminum...Ch. 2 - Tin metal (Sn) and purple iodine (I2) combine to...Ch. 2 - When analyzed, an unknown compound gave these...Ch. 2 - Two general chemistry students working together in...Ch. 2 - To find the empirical formula of tin oxide, you...Ch. 2 - Prob. 165SCQCh. 2 - Prob. 166SCQCh. 2 - The photo here depicts what happens when a coil of...Ch. 2 - A jar contains some number of jelly beans. To find...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please label this HNMRarrow_forwardConsider the following gas chromatographs of Compound A, Compound B, and a mixture of Compounds A and B. Inject A B mixture Area= 9 Area = 5 Area = 3 Area Inject . མི། Inject J2 What is the percentage of Compound B in the the mixture?arrow_forwardRank these according to stability. CH3 H3C CH3 1 CH3 H3C 1 most stable, 3 least stable O 1 most stable, 2 least stable 2 most stable, 1 least stable O2 most stable, 3 least stable O3 most stable, 2 least stable O3 most stable, 1 least stable CH3 2 CH3 CH3 H₂C CH3 3 CH3 CHarrow_forward
- Consider this IR and NMR: INFRARED SPECTRUM TRANSMITTANCE 0.8- 0.6 0.4 0.2 3000 10 9 8 00 HSP-00-541 7 CO 6 2000 Wavenumber (cm-1) сл 5 ppm 4 M Which compound gave rise to these spectra? N 1000 1 0arrow_forwardConsider this reaction (molecular weights are under each compound): HC=CH + 2 HCI --> C2H4Cl 2 MW = 26 36.5 99 If 4.4 g of HC=CH are reacted with 110 mL of a 2.3 M HCI solution, and 6.0 g of product are actually produced, what is the percent yield?arrow_forwardWhat is the name of the major product of this reaction? OH CH3 H₂SO4, heat 1-methylcyclohexene O2-methyl-1-cyclohexene O 3-mthylcyclohexene 1-methyl-2-cyclohexenearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY