
Concept explainers
a) Indole STRUCTURE GIVEN IN QUESTION
Interpretation:
The structure of indole is given. Its skeletal structure is to be drawn.
Concept introduction:
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. The end of a line represents a carbon atom with three hydrogen atoms, CH3; a two-way intersection is a carbon atom with two hydrogen atoms, CH2; a three way intersection is a carbon with one hydrogen, CH; a four way intersection is a carbon with no attached hydrogen. Atoms other than carbon and hydrogen are shown. Nitrogen is trivalent and it can form one double bond and a single bond or three single bonds.
To draw:
The skeletal structure of indole.
Answer to Problem 42AP
The skeletal structure of indole is
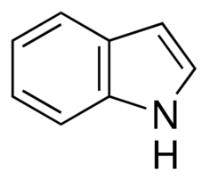
Explanation of Solution
The molecular formula of indole is C8H7N. It has bicyclic structure consisting of a six membered benzene ring fused to a five membered pyrrole ring. In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. A three way intersection is a carbon with one hydrogen, CH; a four way intersection is a carbon with no attached hydrogen. Atoms other than carbon and hydrogen are shown. Nitrogen is trivalent and it is in a three way intersection as NH.
The skeletal structure of indole is

b) 1,3- pentadiene
Interpretation:
The structure of 1,3- pentadiene is given. Its skeletal structure is to be drawn.
Concept introduction:
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. The end of a line represents a carbon atom with three hydrogen atoms, CH3; a two-way intersection is a carbon atom with two hydrogen atoms, CH2; a three way intersection is a carbon with one hydrogen, CH; a four way intersection is a carbon with no attached hydrogen. Atoms other than carbon and hydrogen are shown.
To draw:
The skeletal structure of 1,3- pentadiene.
Answer to Problem 42AP
The skeletal structure of 1,3- pentadiene is
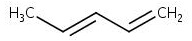
Explanation of Solution
The molecular formula of 1,3- pentadiene is C5H8. It has two double bonds, one between C1-C2 and another between C3-C4. In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. The end of a line represents a carbon atom with three hydrogen atoms, CH3; a two-way intersection is a carbon atom with two hydrogen atoms, CH2; a three way intersection is a carbon with one hydrogen, CH. Based on these concepts the structure of 1,3- pentadiene can be written.
The skeletal structure of 1,3- pentadiene is

c) 1,2- dichloro cyclopentane
Interpretation:
The structure of 1,2- dichloro cyclopentane is given. Its skeletal structure is to be drawn.
Concept introduction:
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. The end of a line represents a carbon atom with three hydrogen atoms, CH3; a two-way intersection is a carbon atom with two hydrogen atoms, CH2; a three way intersection is a carbon with one hydrogen, CH; a four way intersection is a carbon with no attached hydrogen. Atoms other than carbon and hydrogen are shown.
To draw:
The skeletal structure of 1,2- dichloro cyclopentane.
Answer to Problem 42AP
The skeletal structure of 1,2- dichloro cyclopentane is
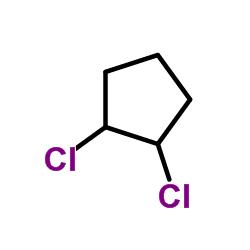
Explanation of Solution
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. Atoms other than carbon and hydrogen are shown. The molecular formula of 1,2- dichlorocyclopentene is C5H8Cl2. A cyclopentane ring is made up of five methylene groups. In 1,2- dichlorocyclopentene the two chlorine atoms are bonded to the first and second carbons replacing one hydrogen on each. They are shown in the skeletal structure.
The skeletal structure of 1,2- dichloro cyclopentane is

d) benzoquinone
Interpretation:
The structure of benzoquinone is given. Its skeletal structure is to be drawn.
Concept introduction:
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. Atoms other than carbon and hydrogen are shown.
To draw:
The skeletal structure of benzoquinone.
Answer to Problem 42AP
The skeletal structure of benzoquinone is
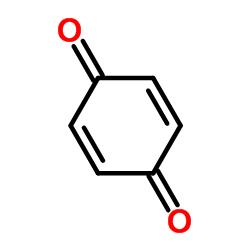
Explanation of Solution
In skeletal structures the carbon atoms are not usually shown. Instead a carbon is assumed to be at each intersection of two lines and at the end of each line. The hydrogen atoms bonded to carbons are also not shown. The correct number of hydrogen atoms for each carbon atom is assigned keeping in mind that carbon has a valence of 4. Atoms other than carbon and hydrogen are shown. The molecular formula of benzoquinone is C6H4O2. The six carbons form a six membered ring with first and fourth carbons as carbonyl groups. The other four carbons have one hydrogen each.
The skeletal structure of benzoquinone is

Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
- Can you explain how I get these here and show the steps plz?arrow_forwardGive the IUPAC name for this compound Hydrocarbon Condensed Formulas Hint C2H5 CH2CH3 expand that in all the formula Part A: (CH3)2CHCH(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part B: CH2=C(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part C: (CH3)2C=CHC(C2H5)=CH2 Give the IUPAC name for this compound. Part D: CH3C=CCH(C2H5)2 Give the IUPAC name for this compound. Part E: (CH3)3CC=CCH2CH=C(CH3)2arrow_forwardSelect/ Match the correct letter from the image below for the IUPAC names given below: A B C D 3 E F G H K L Part 1. 4-methylheptane For example.mmmm Answer Letter H _for part 1 Part 2. 2,4-dimethylhexane Part 3. 2,3-dimethylpentane Part 4. 2,2-dimethylhexane Part 5. 2-ethyl-1,1,3,3-tetramethylcyclopentane Part 6. 3-ethyl-2-methylpentanearrow_forward
- Can u show the process as to how to get these?arrow_forwardSketch the expected 'H NMR spectra for the following compound. Label all of the H's in the structure and the corresponding signal for the spectra you sketch. Make sure you include the integration value and the splitting pattern for each signal Indicate how many signals you would expect in the 13C NMRarrow_forwardUse IUPAC naming rules to name the following hydrocarbon compounds: CH2-CH3 | a) CH-CH-CH2-CH-CH-CH3 b) | CH2 CH3 | CH3 CH3 \ / C=C H 1 H CH2-CH3 c) d) CH=C-CH3 e) CH3-CH2-CH2-CH=CH-CH3 f) CH2=CH-CH2-CH=CH-CH3 g) CH3-CH2-C = C-CH2-CH3 h)arrow_forward
- Q5 Name the following : a. b. C. d. e.arrow_forward25. Predict the major product of the following reaction. 1 equivalent of each of the starting materials was used. H₂C CH3 CH3 H3C H3C H3C. CH2 + H3C. heat CH3 CH H.C. CH3 H.C H.C CH3 CH CH3 CH3 A B C Earrow_forwardFind chemical structures based on the below information. a) Chemical formula C6H8O Compound is aromatic plus has two 1H NMR peaks that integrated for 3 each that are singlets (it could have more peaks in the 1H NMR b) Chemical Formula: C6H100 Compounds is conjugated 'H NMR has a signal that integrates for 6 and is a doublet IR spectra has a signal at 1730 cm-1arrow_forward
- Jaslev Propose a synthesis of the following starting from benzene and any other reagents and chemicals. No mechanisms are required. Indicate the condition for each step plus the major product for each step. More than two steps are required. Step 1 Step 2 مہد Brarrow_forwardPart C: The line formula for another branched alkane is shown below. i. In the IUPAC system what is the root or base name of this compound? ii. How many alkyl substituents are attached to the longest chain? iii. Give the IUPAC name for this compound.arrow_forwardPart D: Draw the Structural Formula for 4-ethyl-2-methylhexane Part E. Draw the Structural Formula for 1-chloro-3,3-diethylpentane (Chloro = Cl)arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning



