
a)
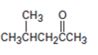
Interpretation:
How to prepare 4-methylpentan-2-one from 4-methyl-3-pentene-2-one is to be shown.
Concept introduction:
The double bond in an unsaturated
To show:
How to prepare 4-methylpentan-2-one from 4-methyl-3-pentene-2-one.
b)
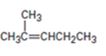
Interpretation:
How to prepare 2-methylpent-2-ene from 4-methyl-3-pentene-2-one is to be shown.
Concept introduction:
The carbonyl group in a unsaturated ketone can be reduced to –CH2 without affecting the double bond by treating it with hydrazine in the presence of KOH (Wolf-Kishner reduction).
To show:
How to prepare 2-methylpent-2-ene from 4-methyl-3-pentene-2-one.
c)
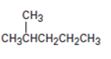
Interpretation:
How to prepare 2-methylpentane from 4-methyl-3-pentene-2-one is to be shown.
Concept introduction:
The double bond in an unsaturated ketone is reduced without affecting the carbonyl group by treating it with H2/Pd. The carbonyl group of the saturated ketone formed can be converted to –CH2 group by Wolff-Kishner reduction.
To show:
How to prepare 2-methylpentane from 4-methyl-3-pentene-2-one.
Trending nowThis is a popular solution!

Chapter 19 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- Identify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forward
- a. H3C CH3 H, 1.0 equiv. Br2arrow_forwardH3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forward
- in the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forward
