
Physics for Scientists and Engineers with Modern Physics
4th Edition
ISBN: 9780131495081
Author: Douglas C. Giancoli
Publisher: Addison-Wesley
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19.7, Problem 1EE
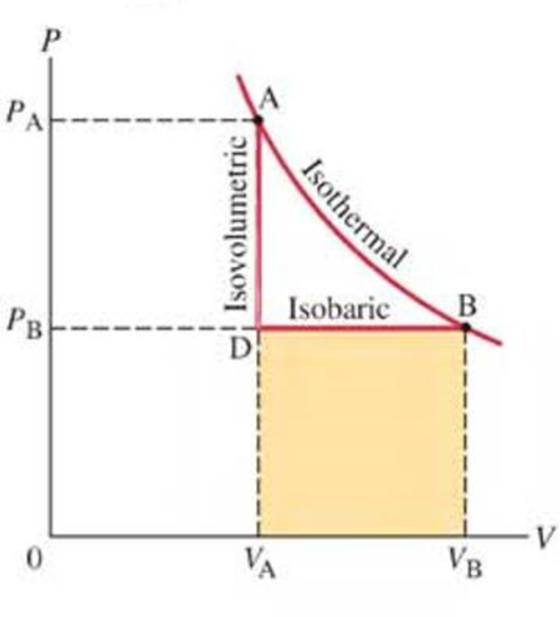
Is the work done by the gas in process ADB of Fig. 19–12 greater than, less than, or equal to the work done in the isothermal process AB?
FIGURE 19-12 Process ADB consists of an isovolumetric (AD) and an isobaric (DB) process.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Question 3 of 17
L
X
L
L
T
0.5/
In the figure above, three uniform thin rods, each of length L, form
an inverted U. The vertical rods each have a mass m; the horizontal
rod has a mass 3m.
NOTE: Express your answer in terms of the variables given.
(a) What is the x coordinate of the system's center of mass?
xcom
L
2
(b) What is the y coordinate of the system's center of mass?
Ycom
45
L
X
Q Search
MD
bp
N
Sketch the harmonic on graphing paper.
Exercise 1:
(a) Using the explicit formulae derived in the lectures for the (2j+1) × (2j + 1) repre-
sentation matrices Dm'm, (J/h), derive the 3 × 3 matrices corresponding to the case
j = 1.
(b) Verify that they satisfy the so(3) Lie algebra commutation relation:
[D(Î₁/ħ), D(Î₂/h)]m'm₁ = iƊm'm² (Ĵ3/h).
(c) Prove the identity
3
Dm'm,(β) = Σ (D(Ρ)D(Ρ))m'¡m; ·
i=1
Chapter 19 Solutions
Physics for Scientists and Engineers with Modern Physics
Ch. 19.2 - Return to the Chapter-Opening Question, page 496,...Ch. 19.5 - Prob. 1BECh. 19.5 - How much more ice at 10C would be needed in...Ch. 19.6 - What would be the internal energy change in...Ch. 19.7 - Is the work done by the gas in process ADB of Fig....Ch. 19.7 - In Example 1910, if the heat lost from the gas in...Ch. 19.10 - Fanning yourself on a hot day cools you by (a)...Ch. 19 - What happens to the work done on a jar of orange...Ch. 19 - Prob. 2QCh. 19 - Prob. 3Q
Ch. 19 - Prob. 4QCh. 19 - Prob. 5QCh. 19 - Why does water in a canteen stay cooler if the...Ch. 19 - Explain why burns caused by steam at 100C on the...Ch. 19 - Prob. 8QCh. 19 - Will potatoes cook faster if the water is boiling...Ch. 19 - Prob. 10QCh. 19 - Prob. 11QCh. 19 - Use the conservation of energy to explain why the...Ch. 19 - In an isothermal process, 3700 J of work is done...Ch. 19 - Explorers on failed Arctic expeditions have...Ch. 19 - Why is wet sand at the beach cooler to walk on...Ch. 19 - When hot-air furnaces are used to heat a house,...Ch. 19 - Is it possible for the temperature of a system to...Ch. 19 - Discuss how the first law of thermodynamics can...Ch. 19 - Explain in words why CP is greater than CV.Ch. 19 - Prob. 20QCh. 19 - An ideal monatomic gas is allowed to expand slowly...Ch. 19 - Ceiling fans are sometimes reversible, so that...Ch. 19 - Goose down sleeping bags and parkas are often...Ch. 19 - Microprocessor chips nowadays have a heat sink...Ch. 19 - Sea breezes are often encountered on sunny days at...Ch. 19 - The Earth cools off at night much more quickly...Ch. 19 - Explain why air-temperature readings are always...Ch. 19 - A premature baby in an incubator can be...Ch. 19 - Prob. 29QCh. 19 - A 22C day is warm, while a swimming pool at 22C...Ch. 19 - Prob. 32QCh. 19 - Prob. 33QCh. 19 - Prob. 34QCh. 19 - Prob. 35QCh. 19 - An emergency blanket is a thin shiny...Ch. 19 - Explain why cities situated by the ocean tend to...Ch. 19 - (I) To what temperature will 8700 J of heat raise...Ch. 19 - Prob. 2PCh. 19 - Prob. 3PCh. 19 - (II) A British thermal unit (Btu) is a unit of...Ch. 19 - Prob. 5PCh. 19 - Prob. 6PCh. 19 - (I) An automobile cooling system holds 18 L of...Ch. 19 - Prob. 8PCh. 19 - (II) (a) How much energy is required to bring a...Ch. 19 - Prob. 10PCh. 19 - Prob. 11PCh. 19 - (II) A hot iron horseshoe (mass = 0.40kg), just...Ch. 19 - (II) A 31.5-g glass thermometer reads 23.6C before...Ch. 19 - Prob. 14PCh. 19 - (II) When a 290-g piece of iron at 180C is placed...Ch. 19 - (II) The heat capacity. C, of an object is defined...Ch. 19 - (II) The 1.20-kg head of a hammer has a speed of...Ch. 19 - (I) How much heat is needed to melt 26.50kg of...Ch. 19 - (I) During exercise, a person may give off 180...Ch. 19 - (II) A 35g ice cube at its melting point is...Ch. 19 - (II) High-altitude mountain climbers do not eat...Ch. 19 - (II) An iron boiler of mass 180 kg contains 730kg...Ch. 19 - (II) In a hot days race, a bicyclist consumes 8.0...Ch. 19 - (II) The specific heat of mercury is 138 J/kg C....Ch. 19 - Prob. 25PCh. 19 - (II) A 58-kg ice-skater moving at 7.5 m/s glides...Ch. 19 - (I) Sketch a PV diagram of the following process:...Ch. 19 - (I) A gas is enclosed in a cylinder fitted with a...Ch. 19 - (II) The pressure in an ideal gas is cut in half...Ch. 19 - (II) A 1.0-L volume of air initially at 3.5 atm of...Ch. 19 - (II) Consider the following two-step process. Heat...Ch. 19 - (II) The PV diagram in Fig. 1931 shows two...Ch. 19 - (II) Suppose 2.60 mol of an ideal gas of volume V1...Ch. 19 - (II) In an engine, an almost ideal gas is...Ch. 19 - (II) One and one-half moles of an ideal monatomic...Ch. 19 - (II) Determine (a) the work done and (b) the...Ch. 19 - (II) How much work is done by a pump to slowly...Ch. 19 - (II) When a gas is taken from a to c along the...Ch. 19 - (III) In the process of taking a gas from state a...Ch. 19 - (III) Suppose a gas is taken clockwise around the...Ch. 19 - (III) Determine the work done by 1.00 mol of a van...Ch. 19 - (I) What is the internal energy of 4.50 mol of an...Ch. 19 - Prob. 43PCh. 19 - Prob. 44PCh. 19 - Prob. 45PCh. 19 - What gas is it? (II) Show that the work done by n...Ch. 19 - (II) An audience of 1800 fills a concert hall of...Ch. 19 - Prob. 48PCh. 19 - Prob. 49PCh. 19 - (III) A 1.00-mol sample of an ideal diatomic gas...Ch. 19 - (I) A 1.00-mol sample of an ideal diatomic gas,...Ch. 19 - (II) Show, using Eqs. 196 and 1915, that the work...Ch. 19 - (III) A 3.65-mol sample of an ideal diatomic gas...Ch. 19 - (II) An ideal monatomic gas, consisting of 2.8 mol...Ch. 19 - (III) A 1.00-mol sample of an ideal monatomic gas,...Ch. 19 - (III) Consider a parcel of air moving to a...Ch. 19 - Prob. 57PCh. 19 - (I) One end of a 45-cm-long copper rod with a...Ch. 19 - (II) How long does it take the Sun to melt a block...Ch. 19 - (II) Heat conduction to skin. Suppose 150 W of...Ch. 19 - (II) A ceramic teapot ( = 0.70) and a shiny one (...Ch. 19 - (II) A copper rod and an aluminum rod of the same...Ch. 19 - Prob. 63PCh. 19 - Prob. 64PCh. 19 - (III) A house thermostat is normally set to 22C,...Ch. 19 - (III) Approximately how long should it take 9.5 kg...Ch. 19 - (III) A cylindrical pipe has inner radius R1 and...Ch. 19 - (III) Suppose the insulating qualities of the wall...Ch. 19 - Prob. 69GPCh. 19 - (a) Find the total power radiated into space by...Ch. 19 - Prob. 71GPCh. 19 - A mountain climber wears a goose-down jacket 3.5...Ch. 19 - Prob. 73GPCh. 19 - Estimate the rate at which heat can he conducted...Ch. 19 - A marathon runner has an average metabolism rate...Ch. 19 - A house has well-insulated walls 19.5 cm thick...Ch. 19 - In a typical game of squash (Fig. 19-36), two...Ch. 19 - A bicycle pump is a cylinder 22 cm long and 3.0 cm...Ch. 19 - Prob. 79GPCh. 19 - The temperature within the Earths crust increases...Ch. 19 - An ice sheet forms on a lake. The air above the...Ch. 19 - An iron meteorite melts when it enters the Earths...Ch. 19 - A scuba diver releases a 3.60-cm-diameter...Ch. 19 - A reciprocating compressor is a device that...Ch. 19 - The temperature of the glass surface of a 75-W...Ch. 19 - Suppose 3.0 mol of neon (an ideal monatomic gas)...Ch. 19 - At very low temperatures, the molar specific heat...Ch. 19 - A diesel engine accomplishes ignition without a...Ch. 19 - When 6.30 105 J of heat is added to a gas...Ch. 19 - In a cold environment, a person can lose heat by...Ch. 19 - Prob. 91GP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Which culture uses NAD+? Use the following choices to answer questions. a. E. coli growing in glucose broth at ...
Microbiology: An Introduction
1. Which is a function of the skeletal system? (a) support, (b) hematopoietic site, (c) storage, (d) providing ...
Anatomy & Physiology (6th Edition)
59. The 0.20 kg puck on the frictionless, horizontal table in Figure P6.59 is connected by a string through a h...
College Physics: A Strategic Approach (3rd Edition)
With what geologic feature are the earthquakes in the mid-Atlantic associated?
Applications and Investigations in Earth Science (9th Edition)
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Level 2: Application/Analysis 4. Nitrifying bactcria participatc in the nitrogen cycle mainly by (A) converting...
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Sketch the harmonic.arrow_forwardFor number 11 please sketch the harmonic on graphing paper.arrow_forward# E 94 20 13. Time a) What is the frequency of the above wave? b) What is the period? c) Highlight the second cycle d) Sketch the sine wave of the second harmonic of this wave % 7 & 5 6 7 8 * ∞ Y U 9 0 0 P 150arrow_forward
- Show work using graphing paperarrow_forwardCan someone help me answer this physics 2 questions. Thank you.arrow_forwardFour capacitors are connected as shown in the figure below. (Let C = 12.0 μF.) a C 3.00 με Hh. 6.00 με 20.0 με HE (a) Find the equivalent capacitance between points a and b. 5.92 HF (b) Calculate the charge on each capacitor, taking AV ab = 16.0 V. 20.0 uF capacitor 94.7 6.00 uF capacitor 67.6 32.14 3.00 µF capacitor capacitor C ☑ με με The 3 µF and 12.0 uF capacitors are in series and that combination is in parallel with the 6 μF capacitor. What quantity is the same for capacitors in parallel? μC 32.14 ☑ You are correct that the charge on this capacitor will be the same as the charge on the 3 μF capacitor. μCarrow_forward
- In the pivot assignment, we observed waves moving on a string stretched by hanging weights. We noticed that certain frequencies produced standing waves. One such situation is shown below: 0 ст Direct Measurement ©2015 Peter Bohacek I. 20 0 cm 10 20 30 40 50 60 70 80 90 100 Which Harmonic is this? Do NOT include units! What is the wavelength of this wave in cm with only no decimal places? If the speed of this wave is 2500 cm/s, what is the frequency of this harmonic (in Hz, with NO decimal places)?arrow_forwardFour capacitors are connected as shown in the figure below. (Let C = 12.0 µF.) A circuit consists of four capacitors. It begins at point a before the wire splits in two directions. On the upper split, there is a capacitor C followed by a 3.00 µF capacitor. On the lower split, there is a 6.00 µF capacitor. The two splits reconnect and are followed by a 20.0 µF capacitor, which is then followed by point b. (a) Find the equivalent capacitance between points a and b. µF(b) Calculate the charge on each capacitor, taking ΔVab = 16.0 V. 20.0 µF capacitor µC 6.00 µF capacitor µC 3.00 µF capacitor µC capacitor C µCarrow_forwardTwo conductors having net charges of +14.0 µC and -14.0 µC have a potential difference of 14.0 V between them. (a) Determine the capacitance of the system. F (b) What is the potential difference between the two conductors if the charges on each are increased to +196.0 µC and -196.0 µC? Varrow_forward
- Please see the attached image and answer the set of questions with proof.arrow_forwardHow, Please type the whole transcript correctly using comma and periods as needed. I have uploaded the picture of a video on YouTube. Thanks,arrow_forwardA spectra is a graph that has amplitude on the Y-axis and frequency on the X-axis. A harmonic spectra simply draws a vertical line at each frequency that a harmonic would be produced. The height of the line indicates the amplitude at which that harmonic would be produced. If the Fo of a sound is 125 Hz, please sketch a spectra (amplitude on the Y axis, frequency on the X axis) of the harmonic series up to the 4th harmonic. Include actual values on Y and X axis.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY