
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
3rd Edition
ISBN: 9781118203774
Author: Klein
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 19.6, Problem 22CC
Interpretation Introduction
Interpretation:
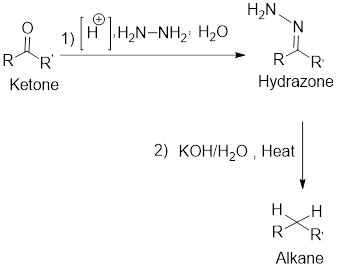
Along with the mechanism, the product has to be predicted for the given two-step reaction.
Concept Introduction:
Wolf-Kishner reduction:
It is the reduction of
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(2 pts) WSe2 is an ionic compound semiconductor that can be made to be p-type or n-type.What must happen to the chemical composition for it to be p-type? What must happen tothe chemical composition for it to be n-type?
8. (2 pts) Silicon semiconductors have a bandgap of
1.11 eV. What is the longest photon wavelength that
can promote an electron from the valence band to
the conduction band in a silicon-based
photovoltaic solar cell? Show all work.
E = hv = hc/λ
h = 6.626 x 10-34 Js
c = 3.00 x 108 m/s
1 eV
1.602 x 10-19 J
A solution containing 100.0 mL of 0.155 M EDTA buffered to pH 10.00 was titrated with 100.0 mL of 0.0152 M Hg(ClO4)2 in a cell:
calomel electrode (saturated)//titration solution/Hg(l)
Given the formation constant of Hg(EDTA)2-, logKf= 21.5, and alphaY4-=0.30, find out the cell voltage E.
Hg2+(aq) + 2e- = Hg(l) E0= 0.852 V
E' (calomel electrode, saturated KCl) = 0.241 V
Chapter 19 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
Ch. 19.2 - Prob. 1LTSCh. 19.2 - Prob. 1PTSCh. 19.2 - Prob. 2PTSCh. 19.2 - APPLY the skill
Compounds with two ketone groups...Ch. 19.2 - Prob. 4ATSCh. 19.3 - Prob. 5CCCh. 19.4 - Prob. 6CCCh. 19.5 - Prob. 7CCCh. 19.5 - Prob. 2LTSCh. 19.5 - Prob. 8PTS
Ch. 19.5 - Prob. 9ATSCh. 19.5 - Prob. 10CCCh. 19.5 - Prob. 11CCCh. 19.5 - Prob. 12CCCh. 19.5 - Prob. 13CCCh. 19.6 - Prob. 3LTSCh. 19.6 - Prob. 14PTSCh. 19.6 - Prob. 15PTSCh. 19.6 - Prob. 16ATSCh. 19.6 - Prob. 17CCCh. 19.6 - Prob. 18CCCh. 19.6 - Prob. 20PTSCh. 19.6 - Prob. 21ATSCh. 19.6 - Prob. 22CCCh. 19.7 - Prob. 5LTSCh. 19.7 - Prob. 23PTSCh. 19.7 - Prob. 24ATSCh. 19.7 - Prob. 25CCCh. 19.8 - Prob. 26CCCh. 19.8 - Prob. 27CCCh. 19.9 - Prob. 28CCCh. 19.9 - Prob. 29CCCh. 19.10 - Prob. 30CCCh. 19.10 - Prob. 31CCCh. 19.10 - Prob. 32CCCh. 19.10 - Prob. 33CCCh. 19.10 - Prob. 6LTSCh. 19.10 - Prob. 34PTSCh. 19.10 - Prob. 35PTSCh. 19.10 - Prob. 36ATSCh. 19.10 - Prob. 37ATSCh. 19.10 - Prob. 38CCCh. 19.11 - Prob. 39CCCh. 19.12 - Prob. 7LTSCh. 19.12 - Prob. 40PTSCh. 19.12 - Prob. 41ATSCh. 19.13 - Prob. 42CCCh. 19 - Prob. 43PPCh. 19 - Prob. 44PPCh. 19 - Prob. 45PPCh. 19 - Prob. 46PPCh. 19 - Prob. 47PPCh. 19 - Prob. 48PPCh. 19 - Prob. 49PPCh. 19 - Prob. 50PPCh. 19 - Prob. 51PPCh. 19 - Prob. 52PPCh. 19 - Prob. 53PPCh. 19 - Prob. 54PPCh. 19 - Prob. 55PPCh. 19 - Prob. 56PPCh. 19 - Prob. 57PPCh. 19 - Prob. 58PPCh. 19 - Prob. 59PPCh. 19 - Prob. 60PPCh. 19 - Predict the major product(s) obtained when each of...Ch. 19 - Prob. 62PPCh. 19 - Prob. 63PPCh. 19 - Prob. 64PPCh. 19 - Prob. 65PPCh. 19 - Prob. 66PPCh. 19 - Prob. 67PPCh. 19 - Prob. 68PPCh. 19 - Prob. 69PPCh. 19 - Prob. 70PPCh. 19 - Prob. 71PPCh. 19 - Prob. 72PPCh. 19 - Prob. 73PPCh. 19 - Prob. 74IPCh. 19 - Prob. 75IPCh. 19 - Prob. 76IPCh. 19 - Prob. 77IPCh. 19 - Prob. 78IPCh. 19 - Prob. 79IPCh. 19 - Prob. 80IPCh. 19 - Prob. 81IPCh. 19 - Prob. 83IPCh. 19 - Prob. 84IPCh. 19 - Prob. 85IPCh. 19 - Prob. 86IPCh. 19 - Prob. 87IPCh. 19 - Prob. 88IPCh. 19 - Prob. 89IPCh. 19 - Prob. 90IPCh. 19 - Prob. 91IPCh. 19 - Prob. 92IPCh. 19 - Prob. 93IPCh. 19 - Prob. 94CPCh. 19 - Prob. 95CPCh. 19 - Treatment of the following ketone with LiAIHa...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- From the following reduction potentials I2 (s) + 2e- = 2I- (aq) E0= 0.535 V I2 (aq) + 2e- = 2I- (aq) E0= 0.620 V I3- (aq) + 2e- = 3I- (aq) E0= 0.535 V a) Calculate the equilibrium constant for I2 (aq) + I- (aq) = I3- (aq). b) Calculate the equilibrium constant for I2 (s) + I- (aq) = I3- (aq). c) Calculate the solubility of I2 (s) in water.arrow_forward2. (3 pts) Consider the unit cell for the spinel compound, CrFe204. How many total particles are in the unit cell? Also, show how the number of particles and their positions are consistent with the CrFe204 stoichiometry - this may or may not be reflected by the particle colors in the diagram. (HINT: In the diagram, the blue particle is in an interior position while each red particle is either in a corner or face position.)arrow_forwardFrom the following potentials, calculate the activity of Cl- in saturated KCl. E0 (calomel electrode)= 0.268 V E (calomel electrode, saturated KCl)= 0.241 Varrow_forward
- Calculate the voltage of each of the following cells. a) Fe(s)/Fe2+ (1.55 x 10-2 M)//Cu2+ (6.55 x 10-3 M)/Cu(s) b) Pt, H2 (0.255 bar)/HCl (4.55 x 10-4 M), AgCl (sat'd)/Ag Fe2+ +2e- = Fe E0= -0.44 V Cu2+ + 2e- = Cu E0= 0.337 V Ag+ + e- = Ag E0= 0.799 V AgCl(s) + e- = Ag(s) + Cl- E0= 0.222 V 2H+ + 2e- = H2 E0= 0.000 Varrow_forwardA solution contains 0.097 M Ce3+, 1.55x10-3 M Ce4+, 1.55x10-3 M Mn2+, 0.097 M MnO4-, and 1.00 M HClO4 (F= 9.649 x 104 C/mol). a) Write a balanced net reaction that can occur between species in this solution. b) Calculate deltaG0 and K for the reaction. c) Calculate E and deltaG for the conditions given. Ce4+ + e- = Ce3+ E0= 1.70 V MnO4- + 8H+ + 5e- = Mn2+ + 4H2O E0= 1.507 Varrow_forward1. Provide a step-by-step mechanism for formation of ALL STEREOISOMERS in the following reaction. Na HCO3 (Sodium bicarbonate, baking soda) is not soluble in CH2Cl2. The powder is a weak base used to neutralize strong acid (pKa < 0) produced by the reaction. Redraw the product to show the configuration(s) that form at C-2 and C-4. Br2 OH CH2Cl2 Na* HCO3 Br HO OH + Na Br +arrow_forward
- 2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O2/HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI + enant OH Solvent Reagent(s) Solvent Reagent(s)arrow_forwardGermanium (Ge) is a semiconductor with a bandgap of 2.2 eV. How could you dope Ge to make it a p-type semiconductor with a larger bandgap? Group of answer choices It is impossible to dope Ge and have this result in a larger bandgap. Dope the Ge with silicon (Si) Dope the Ge with gallium (Ga) Dope the Ge with phosphorus (P)arrow_forwardWhich of the following semiconductors would you choose to have photons with the longest possible wavelengths be able to promote electrons to the semiconductor's conduction band? Group of answer choices Si Ge InSb CdSarrow_forward
- Which of the following metals is the only one with all of its bands completely full? Group of answer choices K Na Ca Alarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); Reagents: H₂O (B); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI OH - α-α Br + enant Solvent Reagent(s) Solvent Reagent(s)arrow_forwardBased on concepts from Lecture 3-5, which of the following ionic compounds should be most soluble in water? Group of answer choices MgO BeO CaO BaOarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License