
Conceptual Phy. Sci. - With Access (Custom)
6th Edition
ISBN: 9781323406588
Author: Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 72E
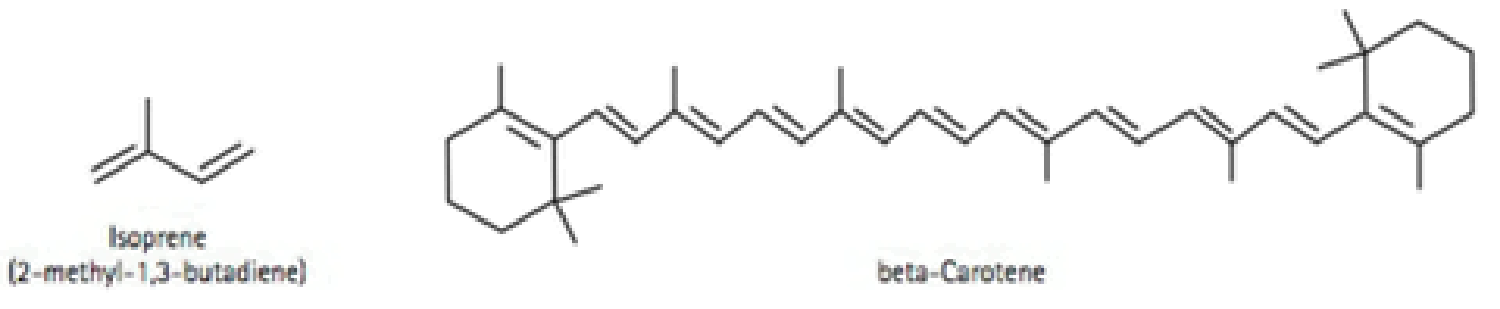
Many of the natural product molecules synthesized by photosynthetic plants are formed by the joining together of isoprene monomers via an addition
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4. Does the period of simple harmonic motion depend on amplitude?
Show that x(t) = A cos (wt) + B sin (wt) is a solution to the differential equation of the mass/spring system
2. List three places besides in springs where Hooke's law applies.
Chapter 19 Solutions
Conceptual Phy. Sci. - With Access (Custom)
Ch. 19 - How do two structural isomers differ from each...Ch. 19 - How are two structural isomers similar to each...Ch. 19 - What physical property of hydrocarbons is used in...Ch. 19 - What types of hydrocarbons are more abundant in...Ch. 19 - To how many atoms is a saturated carbon atom...Ch. 19 - What is the difference between a saturated...Ch. 19 - How many multiple bonds must a hydrocarbon have in...Ch. 19 - What kind of ring do aromatic compounds contain?Ch. 19 - What is a heteroatom?Ch. 19 - Why do heteroatoms make such a difference in the...
Ch. 19 - Why are low-formula-mass alcohols soluble in...Ch. 19 - What distinguishes an alcohol from a phenol?Ch. 19 - What distinguishes an alcohol from an ether?Ch. 19 - Which heteroatom is characteristic of an amine?Ch. 19 - Do amines tend to be acidic, neutral, or basic?Ch. 19 - Are alkaloids found in nature?Ch. 19 - What are some examples of alkaloids?Ch. 19 - Which elements make up the carbonyl group?Ch. 19 - How are ketones and aldehydes related to each...Ch. 19 - How are amides and carboxylic acids related to...Ch. 19 - From what naturally occurring compound is aspirin...Ch. 19 - What happens to the double bond of a monomer...Ch. 19 - What is released in the formation of a...Ch. 19 - Why is plastic wrap made of polyvinylidene...Ch. 19 - Prob. 25RCQCh. 19 - Rank the following molecules in order of the phase...Ch. 19 - Rank the following hydrocarbons in order of...Ch. 19 - Rank the following hydrocarbons in order of...Ch. 19 - Rank the following organic molecules in order of...Ch. 19 - Rank the following organic molecules in order of...Ch. 19 - What property of carbon allows for the formation...Ch. 19 - Why does the melting point of hydrocarbons...Ch. 19 - Draw all the structural isomers for hydrocarbons...Ch. 19 - How many structural isomers are shown here?Ch. 19 - According to Figure 19.3, which has the higher...Ch. 19 - The temperatures in a fractionating tower at an...Ch. 19 - Prob. 40ECh. 19 - Do heavier hydrocarbons tend to produce more or...Ch. 19 - What do these two structures have in common?Ch. 19 - What do the compounds cyclopropane and propene...Ch. 19 - What are the chemical formulas for the following...Ch. 19 - Prob. 45ECh. 19 - Prob. 46ECh. 19 - Identify the following functional groups in this...Ch. 19 - What must be added to a double bond to transform...Ch. 19 - What do phenols and carboxylic acids have in...Ch. 19 - What is the difference between a ketone and an...Ch. 19 - Prob. 51ECh. 19 - Prob. 52ECh. 19 - What is the percent volume of water in 80-proof...Ch. 19 - One of the skin-irritating components of poison...Ch. 19 - Cetyl alcohol, C16H34O, is a common ingredient of...Ch. 19 - A common inactive ingredient in products such as...Ch. 19 - A common inactive ingredient in products such as...Ch. 19 - The phosphoric acid salt of caffeine has the...Ch. 19 - Prob. 59ECh. 19 - In water, does the following molecule act as an...Ch. 19 - If you saw the label phenylephrine-HCl on a...Ch. 19 - The amino acid lysine is shown below. What...Ch. 19 - Prob. 63ECh. 19 - Suggest an explanation why aspirin has a sour...Ch. 19 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 19 - What products are formed upon the reaction of...Ch. 19 - The disodium salt of ethylenediaminetetraacetic...Ch. 19 - Would you expect polypropylene to be more dense or...Ch. 19 - Hydrocarbons release a lot of energy when ignited....Ch. 19 - The polymer styrene-butadiene rubber (SBR), shown...Ch. 19 - Citral and camphor are both 10-carbon odoriferous...Ch. 19 - Many of the natural product molecules synthesized...Ch. 19 - The solvent diethyl ether can be mixed with water...Ch. 19 - Alkaloid salts are not very soluble in the organic...Ch. 19 - Why does the melting point of hydrocarbons...Ch. 19 - How many structural isomers are there for...Ch. 19 - Which contains more hydrogen atoms: a five-carbon...Ch. 19 - Prob. 4RATCh. 19 - Why might a high-formula-mass alcohol be insoluble...Ch. 19 - Alkaloid salts are not very soluble in the organic...Ch. 19 - Explain why caprylic acid, CH3(CH2)6 COOH,...Ch. 19 - How many oxygen atoms are bonded to the carbon of...Ch. 19 - One solution to the problem of our overflowing...Ch. 19 - Which would you expect to be more viscous: a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
In recent years, scientists have discovered hundreds of planets orbiting other stars. Some of these planets are...
College Physics: A Strategic Approach (3rd Edition)
In tomato plants, purple leaf color is controlled by a dominant allele A, and green leaf by a recessive allele ...
Genetic Analysis: An Integrated Approach (3rd Edition)
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Modified True/False 6. __________ Halophiles inhabit extremely saline habitats, such as the Great Salt Lake.
Microbiology with Diseases by Body System (5th Edition)
Which type of cartilage is most plentiful in the adult body?
Anatomy & Physiology (6th Edition)
explain the function of fermentation and the conditions under which it occurs?
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 1. What is the spring constant of a spring that starts 10.0 cm long and extends to 11.4 cm with a 300 g mass hanging from it?arrow_forwardplease help me solve all parts of this question from physics. thanks so much in advance! :)))arrow_forwardA fluid with density 263 kg/m3 flows through a pipe of varying diameter and height. At location 1 the flow speed is 13.5 m/s and the diameter of the pipe is 7.4 cm down to location 2 the pipe diameter is 16.9 cm. Location 1 is 6.3 meters higher than location 2. What is the difference in pressure P2 - P1? Using units in Pascals and use g = 9.81 m/s2.arrow_forward
- The kitchen had a temperature 46 degrees Fahrenheit and was converted it to Kelvin. What is the correct number for this temperature (46 F) on the Kelvin scale?arrow_forwardWater is traveling at a speed of 0.65 m/s through a pipe with a cross-section radius of 0.23 meters. The water enters a section of pipe that has a smaller radius, only 0.11 meters. What is the speed of the water traveling in this narrower section of pipe?arrow_forwardA particular water pipe has a radius of 0.28 meters. If the pipe is completely filled with water, moving with average velocity 0.45 m/s, what is the flow rate of water through the pipe with units of cubic meters of water per second?arrow_forward
- Water is flowing through a horizontal pipe with two segments. In one segment, the water flows at a speed v1 = 4.52 m/s. In the second segment the speed of the water is v2 = 2.38 m/s. Based on Bernoulli's Principle, what is the difference in pressure (P2 - P1) between the two segments? Assume that the density of the water is 997 kg/m3 and give your answer as the number of Pascals (i.e. N/m2).arrow_forwardWater from the faucet is supplied to the hose at a rate of 0.00057 m3/s. At what speed (number of meters per second) does the water exit the nozzle if the cross sectional area of the narrow nozzle is 2.1 x 10-6 m2?arrow_forwardJason Fruits/Indiana University Research Communications Silver/ silver oxide Zinc zinc/oxidearrow_forward
- Car P moves to the west with constant speed v0 along a straight road. Car Q starts from rest at instant 1, and moves to the west with increasing speed. At instant 5, car Q has speed w0 relative to the road (w0 < v0). Instants 1-5 are separated by equal time intervals. At instant 3, cars P and Q are adjacent to one another (i.e., they have the same position). In the reference frame o f the road, at instant 3 i s the speed o f car Q greater than, less than, or equal to the speed of car P? Explain.arrow_forwardCar P moves to the west with constant speed v0 along a straight road. Car Q starts from rest at instant 1, and moves to the west with increasing speed. At instant 5, car Q has speed w0 relative to the road (w0 < v0). Instants 1-5 are separated by equal time intervals.arrow_forwardCar P moves to the west with constant speed v0 along a straight road. Car Q starts from rest at instant 1, and moves to the west with increasing speed. At instant 5, car Q has speed w0 relative to the road (w0 < v0). Instants 1-5 are separated by equal time intervals. Sketch and label a vector diagram illustrating the Galilean transformation of velocities that relates velocity of car P relative to the road, velocity of car Q relative to road, and velocity of car Q relative to car P at instant 3. In the frame of car P, at instant 3 is car Q moving to the west, moving to the east, or at rest? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY