
Conceptual Phy. Sci. - With Access (Custom)
6th Edition
ISBN: 9781323406588
Author: Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 33TAR
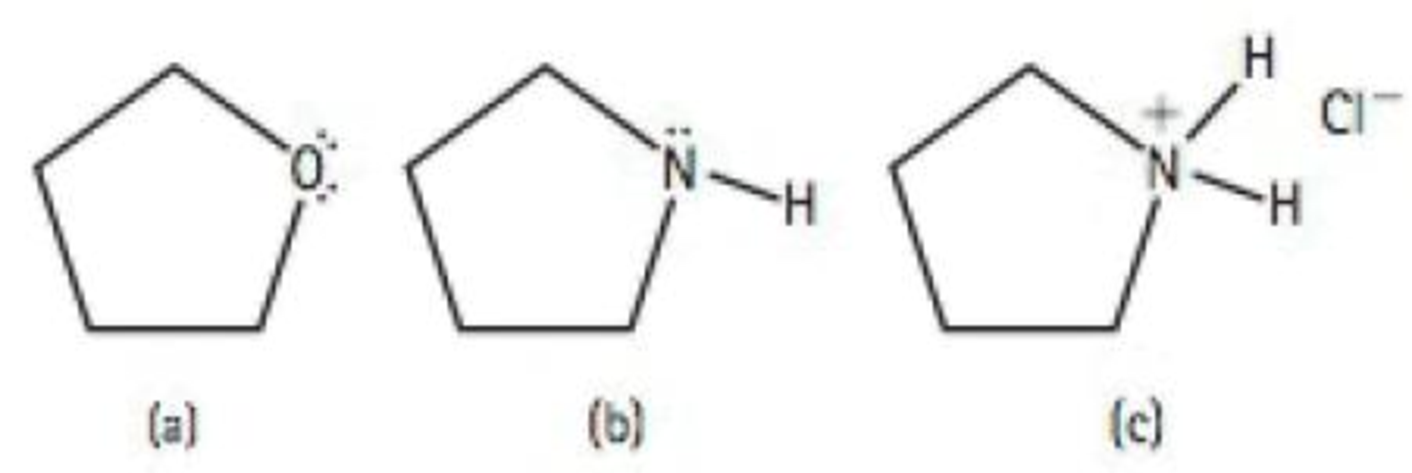
Rank the following organic molecules in order of increasing solubility in water:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V)
ammeter
I =
simple diagram to illustrate the setup for each law- coulombs law and biot savart law
A circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.
Chapter 19 Solutions
Conceptual Phy. Sci. - With Access (Custom)
Ch. 19 - How do two structural isomers differ from each...Ch. 19 - How are two structural isomers similar to each...Ch. 19 - What physical property of hydrocarbons is used in...Ch. 19 - What types of hydrocarbons are more abundant in...Ch. 19 - To how many atoms is a saturated carbon atom...Ch. 19 - What is the difference between a saturated...Ch. 19 - How many multiple bonds must a hydrocarbon have in...Ch. 19 - What kind of ring do aromatic compounds contain?Ch. 19 - What is a heteroatom?Ch. 19 - Why do heteroatoms make such a difference in the...
Ch. 19 - Why are low-formula-mass alcohols soluble in...Ch. 19 - What distinguishes an alcohol from a phenol?Ch. 19 - What distinguishes an alcohol from an ether?Ch. 19 - Which heteroatom is characteristic of an amine?Ch. 19 - Do amines tend to be acidic, neutral, or basic?Ch. 19 - Are alkaloids found in nature?Ch. 19 - What are some examples of alkaloids?Ch. 19 - Which elements make up the carbonyl group?Ch. 19 - How are ketones and aldehydes related to each...Ch. 19 - How are amides and carboxylic acids related to...Ch. 19 - From what naturally occurring compound is aspirin...Ch. 19 - What happens to the double bond of a monomer...Ch. 19 - What is released in the formation of a...Ch. 19 - Why is plastic wrap made of polyvinylidene...Ch. 19 - Prob. 25RCQCh. 19 - Rank the following molecules in order of the phase...Ch. 19 - Rank the following hydrocarbons in order of...Ch. 19 - Rank the following hydrocarbons in order of...Ch. 19 - Rank the following organic molecules in order of...Ch. 19 - Rank the following organic molecules in order of...Ch. 19 - What property of carbon allows for the formation...Ch. 19 - Why does the melting point of hydrocarbons...Ch. 19 - Draw all the structural isomers for hydrocarbons...Ch. 19 - How many structural isomers are shown here?Ch. 19 - According to Figure 19.3, which has the higher...Ch. 19 - The temperatures in a fractionating tower at an...Ch. 19 - Prob. 40ECh. 19 - Do heavier hydrocarbons tend to produce more or...Ch. 19 - What do these two structures have in common?Ch. 19 - What do the compounds cyclopropane and propene...Ch. 19 - What are the chemical formulas for the following...Ch. 19 - Prob. 45ECh. 19 - Prob. 46ECh. 19 - Identify the following functional groups in this...Ch. 19 - What must be added to a double bond to transform...Ch. 19 - What do phenols and carboxylic acids have in...Ch. 19 - What is the difference between a ketone and an...Ch. 19 - Prob. 51ECh. 19 - Prob. 52ECh. 19 - What is the percent volume of water in 80-proof...Ch. 19 - One of the skin-irritating components of poison...Ch. 19 - Cetyl alcohol, C16H34O, is a common ingredient of...Ch. 19 - A common inactive ingredient in products such as...Ch. 19 - A common inactive ingredient in products such as...Ch. 19 - The phosphoric acid salt of caffeine has the...Ch. 19 - Prob. 59ECh. 19 - In water, does the following molecule act as an...Ch. 19 - If you saw the label phenylephrine-HCl on a...Ch. 19 - The amino acid lysine is shown below. What...Ch. 19 - Prob. 63ECh. 19 - Suggest an explanation why aspirin has a sour...Ch. 19 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 19 - What products are formed upon the reaction of...Ch. 19 - The disodium salt of ethylenediaminetetraacetic...Ch. 19 - Would you expect polypropylene to be more dense or...Ch. 19 - Hydrocarbons release a lot of energy when ignited....Ch. 19 - The polymer styrene-butadiene rubber (SBR), shown...Ch. 19 - Citral and camphor are both 10-carbon odoriferous...Ch. 19 - Many of the natural product molecules synthesized...Ch. 19 - The solvent diethyl ether can be mixed with water...Ch. 19 - Alkaloid salts are not very soluble in the organic...Ch. 19 - Why does the melting point of hydrocarbons...Ch. 19 - How many structural isomers are there for...Ch. 19 - Which contains more hydrogen atoms: a five-carbon...Ch. 19 - Prob. 4RATCh. 19 - Why might a high-formula-mass alcohol be insoluble...Ch. 19 - Alkaloid salts are not very soluble in the organic...Ch. 19 - Explain why caprylic acid, CH3(CH2)6 COOH,...Ch. 19 - How many oxygen atoms are bonded to the carbon of...Ch. 19 - One solution to the problem of our overflowing...Ch. 19 - Which would you expect to be more viscous: a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Explain why decomposition rates in a field in Nebraska would differ from the decomposition rates in a field in ...
Biological Science (6th Edition)
41. Humans vary in many ways from one another. Among many minor phenotypic differences are the following five i...
Genetic Analysis: An Integrated Approach (3rd Edition)
The distances you obtained in Question 3 are for only one side of the ridge. Assuming that a ridge spreads equa...
Applications and Investigations in Earth Science (9th Edition)
What is the reducing agent in the following reaction?
2 Br –– (aq) + H2 O2 (aq) + 2 H+ (aq) → Br2 (aq) + 2 H2 ...
Chemistry: The Central Science (14th Edition)
3. What are serous membranes, and what are their functions?
Human Anatomy & Physiology (2nd Edition)
29. For the reaction
determine the expression for the rate of the reaction in terms of the change in concentr...
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- An L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forwardDescribe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forward
- Discuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forwardExplain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward3. An Atwood machine consists of two masses, mA and m B, which are connected by an inelastic cord of negligible mass that passes over a pulley. If the pulley has radius RO and moment of inertia I about its axle, determine the acceleration of the masses mA and m B, and compare to the situation where the moment of inertia of the pulley is ignored. Ignore friction at the axle O. Use angular momentum and torque in this solutionarrow_forward
- A 0.850-m-long metal bar is pulled to the right at a steady 5.0 m/s perpendicular to a uniform, 0.650-T magnetic field. The bar rides on parallel metal rails connected through a 25-Ω, resistor (Figure 1), so the apparatus makes a complete circuit. Ignore the resistance of the bar and the rails. Please explain how to find the direction of the induced current.arrow_forwardFor each of the actions depicted, determine the direction (right, left, or zero) of the current induced to flow through the resistor in the circuit containing the secondary coil. The coils are wrapped around a plastic core. Immediately after the switch is closed, as shown in the figure, (Figure 1) in which direction does the current flow through the resistor? If the switch is then opened, as shown in the figure, in which direction does the current flow through the resistor? I have the answers to the question, but would like to understand the logic behind the answers. Please show steps.arrow_forwardWhen violet light of wavelength 415 nm falls on a single slit, it creates a central diffraction peak that is 8.60 cm wide on a screen that is 2.80 m away. Part A How wide is the slit? ΟΙ ΑΣΦ ? D= 2.7.10-8 Submit Previous Answers Request Answer × Incorrect; Try Again; 8 attempts remaining marrow_forward
- Two complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find θ for (z1-z∗2)/z1+z2∗. Find r and θ for (z1−z2∗)z1z2∗ Please show all stepsarrow_forwardCalculate the center of mass of the hollow cone shown below. Clearly specify the origin and the coordinate system you are using. Z r Y h Xarrow_forward12. If all three collisions in the figure below are totally inelastic, which will cause more damage? (think about which collision has a larger amount of kinetic energy dissipated/lost to the environment? I m II III A. I B. II C. III m m v brick wall ע ע 0.5v 2v 0.5m D. I and II E. II and III F. I and III G. I, II and III (all of them) 2marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY