
Conceptual Phy. Sci. - With Access (Custom)
6th Edition
ISBN: 9781323406588
Author: Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 61E
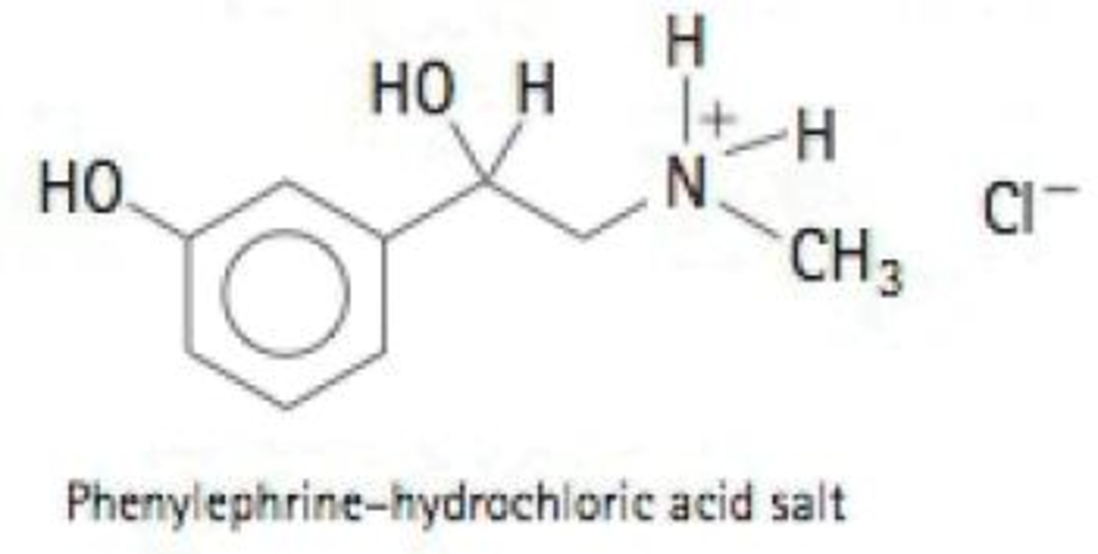
If you saw the label phenylephrine-HCl on a decongest-ant, would you worry that consuming it would expose you to the strong acid hydrochloric acid? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
d
need help with the one i got wrong
Identify which of the following vectors are parallel to A.
Vector
Vector A
70°
60°
20°
AAZA!
30°
20°
20°
70°
20°
20°
70°
20°
Chapter 19 Solutions
Conceptual Phy. Sci. - With Access (Custom)
Ch. 19 - How do two structural isomers differ from each...Ch. 19 - How are two structural isomers similar to each...Ch. 19 - What physical property of hydrocarbons is used in...Ch. 19 - What types of hydrocarbons are more abundant in...Ch. 19 - To how many atoms is a saturated carbon atom...Ch. 19 - What is the difference between a saturated...Ch. 19 - How many multiple bonds must a hydrocarbon have in...Ch. 19 - What kind of ring do aromatic compounds contain?Ch. 19 - What is a heteroatom?Ch. 19 - Why do heteroatoms make such a difference in the...
Ch. 19 - Why are low-formula-mass alcohols soluble in...Ch. 19 - What distinguishes an alcohol from a phenol?Ch. 19 - What distinguishes an alcohol from an ether?Ch. 19 - Which heteroatom is characteristic of an amine?Ch. 19 - Do amines tend to be acidic, neutral, or basic?Ch. 19 - Are alkaloids found in nature?Ch. 19 - What are some examples of alkaloids?Ch. 19 - Which elements make up the carbonyl group?Ch. 19 - How are ketones and aldehydes related to each...Ch. 19 - How are amides and carboxylic acids related to...Ch. 19 - From what naturally occurring compound is aspirin...Ch. 19 - What happens to the double bond of a monomer...Ch. 19 - What is released in the formation of a...Ch. 19 - Why is plastic wrap made of polyvinylidene...Ch. 19 - Prob. 25RCQCh. 19 - Rank the following molecules in order of the phase...Ch. 19 - Rank the following hydrocarbons in order of...Ch. 19 - Rank the following hydrocarbons in order of...Ch. 19 - Rank the following organic molecules in order of...Ch. 19 - Rank the following organic molecules in order of...Ch. 19 - What property of carbon allows for the formation...Ch. 19 - Why does the melting point of hydrocarbons...Ch. 19 - Draw all the structural isomers for hydrocarbons...Ch. 19 - How many structural isomers are shown here?Ch. 19 - According to Figure 19.3, which has the higher...Ch. 19 - The temperatures in a fractionating tower at an...Ch. 19 - Prob. 40ECh. 19 - Do heavier hydrocarbons tend to produce more or...Ch. 19 - What do these two structures have in common?Ch. 19 - What do the compounds cyclopropane and propene...Ch. 19 - What are the chemical formulas for the following...Ch. 19 - Prob. 45ECh. 19 - Prob. 46ECh. 19 - Identify the following functional groups in this...Ch. 19 - What must be added to a double bond to transform...Ch. 19 - What do phenols and carboxylic acids have in...Ch. 19 - What is the difference between a ketone and an...Ch. 19 - Prob. 51ECh. 19 - Prob. 52ECh. 19 - What is the percent volume of water in 80-proof...Ch. 19 - One of the skin-irritating components of poison...Ch. 19 - Cetyl alcohol, C16H34O, is a common ingredient of...Ch. 19 - A common inactive ingredient in products such as...Ch. 19 - A common inactive ingredient in products such as...Ch. 19 - The phosphoric acid salt of caffeine has the...Ch. 19 - Prob. 59ECh. 19 - In water, does the following molecule act as an...Ch. 19 - If you saw the label phenylephrine-HCl on a...Ch. 19 - The amino acid lysine is shown below. What...Ch. 19 - Prob. 63ECh. 19 - Suggest an explanation why aspirin has a sour...Ch. 19 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 19 - What products are formed upon the reaction of...Ch. 19 - The disodium salt of ethylenediaminetetraacetic...Ch. 19 - Would you expect polypropylene to be more dense or...Ch. 19 - Hydrocarbons release a lot of energy when ignited....Ch. 19 - The polymer styrene-butadiene rubber (SBR), shown...Ch. 19 - Citral and camphor are both 10-carbon odoriferous...Ch. 19 - Many of the natural product molecules synthesized...Ch. 19 - The solvent diethyl ether can be mixed with water...Ch. 19 - Alkaloid salts are not very soluble in the organic...Ch. 19 - Why does the melting point of hydrocarbons...Ch. 19 - How many structural isomers are there for...Ch. 19 - Which contains more hydrogen atoms: a five-carbon...Ch. 19 - Prob. 4RATCh. 19 - Why might a high-formula-mass alcohol be insoluble...Ch. 19 - Alkaloid salts are not very soluble in the organic...Ch. 19 - Explain why caprylic acid, CH3(CH2)6 COOH,...Ch. 19 - How many oxygen atoms are bonded to the carbon of...Ch. 19 - One solution to the problem of our overflowing...Ch. 19 - Which would you expect to be more viscous: a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The most plausible hypothesis to explain why species richness is higher in tropical than in temperate regions i...
Campbell Biology (11th Edition)
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
53. This reaction was monitored as a function of time:
A plot of In[A] versus time yields a straight ...
Chemistry: Structure and Properties (2nd Edition)
The number of named species is about __________, but the actual number of species on Earth is estimated to be a...
Biology: Life on Earth (11th Edition)
APPLY 1.2 Express the following quantities in scientific notation
using fundamental SI units of mass and lengt...
Chemistry (7th Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 3aarrow_forward44 please help with the this.arrow_forward4a Which of the following values COULD NOT be a magnitude? Choose all that apply. 626 0 -0.806 8.63 -48.5 72 131 156 4b Px = -1248 & Py = 261. Determine P.P = Qx = -1540 & Qy = 375. Determine Q.Q = 4c. T = 1105 & Ty = 425. Determine the two possible values for Tx. 4d. Uy = -38. Which of the following COULD NOT be the value of U? Choose all that apply. 10 70 72 31 47 0 75 38 4e. R has a magnitude of 165. Which of the following COULD be Rx? Choose all that apply. 165 -171 155 0 -156 -165 172 -130arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Stars and GalaxiesPhysicsISBN:9781305120785Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Stars and GalaxiesPhysicsISBN:9781305120785Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning


Stars and Galaxies
Physics
ISBN:9781305120785
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:9781337399920
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY