
Interpretation:
Products A–E and queen substance for the given reaction chain are to be provided.
Concept introduction:
Grignard reagent reactions with carbonyl groups are treated as acid–base reactions.
The reaction of Grignard reagents with carbonyl groups yields secondary and tertiary alcohols. These reactions are two-step reactions. In the first step, nucleophilic addition of carbonyl group takes place because the Grignard reagent, being nucleophilic, uses its lone pair of electrons to form a bond with a carbon atom. This results in the formation of an alkoxide ion which remains associated with
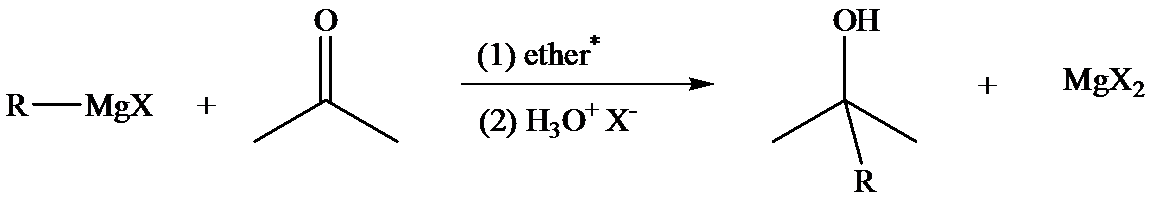
In the second step, addition of aqueous HX causes the protonation of the alkoxide ion, which leads to the formation of the alcohol and
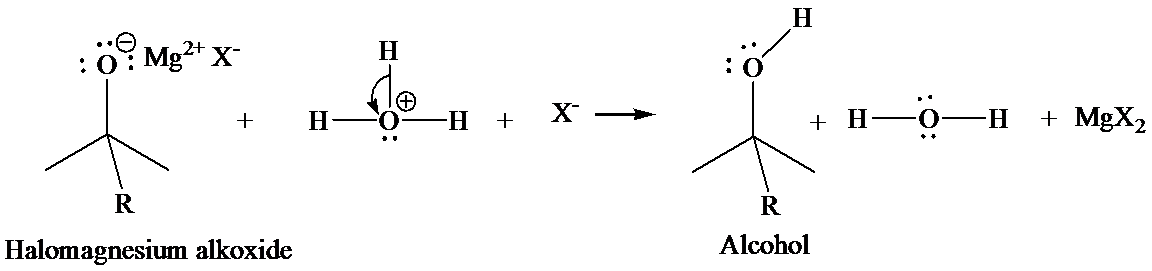
Hydrogenation of unsaturated compounds, such as
Secondary and tertiary alcohols undergo elimination reactions, when they are treated with mineral acids and heat. Dehydration of alcohols via E1 elimination reaction.
Ozonolysis cleaves the cyclic bond of ring hydrocarbons and converts the ring structure to a straight chain. Dimethyl sulfide reduces the intermediate to form a final compound with two carbonyl groups.
When methyl
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
- Predict the major products of this organic reaction. Be sure you use dash and wedge bonds to show stereochemistry where it's important. + ☑ OH 1. TsCl, py .... 文 P 2. t-BuO K Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ( Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. Х : а ค 1arrow_forwardIn the drawing area below, draw the major products of this organic reaction: If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area instead. 1. NaH 2. CH3Br ? Click and drag to start drawing a structure. No reaction. : ☐ Narrow_forward
- + Predict the major product of the following reaction. : ☐ + ☑ ค OH H₂SO4 Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ... OH CI Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. ☐ No Reaction. Click and drag to start drawing a structure. : аarrow_forwardConsider the following reactants: Br Would elimination take place at a significant rate between these reactants? Note for advanced students: by significant, we mean that the rate of elimination would be greater than the rate of competing substitution reactions. yes O no If you said elimination would take place, draw the major products in the upper drawing area. If you said elimination would take place, also draw the complete mechanism for one of the major products in the lower drawing area. If there is more than one major product, you may draw the mechanism that leads to any of them. Major Products:arrow_forward
- Draw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction. OH + ! : ☐ + Х Click and drag to start drawing a structure.arrow_forwardFind one pertinent analytical procedure for each of following questions relating to food safety analysis. Question 1: The presence of lead, mercury and cadmium in canned tuna Question 2: Correct use of food labellingarrow_forwardFormulate TWO key questions that are are specifically in relation to food safety. In addition to this, convert these questions into a requirement for chemical analysis.arrow_forward
- What are the retrosynthesis and forward synthesis of these reactions?arrow_forwardWhich of the given reactions would form meso product? H₂O, H2SO4 III m CH3 CH₂ONa CH3OH || H₂O, H2SO4 CH3 1. LiAlH4, THF 2. H₂O CH3 IVarrow_forwardWhat is the major product of the following reaction? O IV III HCI D = III ა IVarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


