
Concept explainers
Give the number of unpaired electrons in octahedral complexes with strong-field ligands for
(a) Rh3+
(b) Mn3+
(c) Ag+
(d) Pt4+
(e) Au3+
(a)
Interpretation:
The number of unpaired electrons in octahedral complexes with strong field ligands for the metal ion
Concept introduction:
Coordination compounds are those in which a transition metal atom is bonded to a ligand which can be neutral, cation or anion. A transition metal cation has no outer s- electrons available for bonding, rather the inner d -electrons (in case of 3d transition metal elements) are available for making coordinate bonds with the ligands. Electrons are distributed in the five d- orbitals according to Hund’s rule which results in a maximum number of unpaired electrons. The abbreviated electronic configuration of an element depicts the electronic configuration of the elements by making use of noble gas configuration as they have fully-filled electron shells.
Answer to Problem 41QAP
There are zero unpaired electrons in Rh3+.
Explanation of Solution
Rhodium is a 4d transition metal element and its atomic number is 45.Its abbreviated electronic configuration can be written as [Kr] 4d85s1.
When it loses three electrons it leads to the formation of Rh3+ cation, and its abbreviated electronic configuration is written as [Kr] 4d6.
The distribution of electrons in the 4d orbitals when no ligand is present is given as follows:
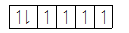
In case of octahedral complexes, the distribution of electrons in the five d-orbitals takes place according to the crystal field theory, according to which in octahedral complexes as the ligand approaches the central metal atom, its d -orbitals get split into lower energy orbitals
Strong field ligands interact directly with the metal d-electrons and causes pairing of the electrons. The compound so formed is a low spin complex.
The distribution of electrons is shown below:
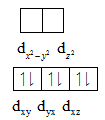
In case of low spin complexes, the unpaired electrons present in the higher energy gets paired with the electrons present in the t2g orbitals. From the above electronic distribution, Rh3+ does not contain any unpaired electrons.
(b)
Interpretation:
The number of unpaired electrons in octahedral complexes with strong field ligands for the metal ion
Concept introduction:
Coordination compounds are those in which a transition metal atom is bonded to a ligand which can be neutral, cation or anion. A transition metal cation has no outer s- electrons available for bonding, rather the inner d -electrons (in case of 3d transition metal elements) are available for making coordinate bonds with the ligands. Electrons are distributed in the five d- orbitals according to Hund’s rule which results in a maximum number of unpaired electrons. The abbreviated electronic configuration of an element depicts the electronic configuration of the elements by making use of noble gas configuration as they have fully-filled electron shells.
Answer to Problem 41QAP
There are two unpaired electrons in Mn3+
Explanation of Solution
Manganese is a 3d transition metal element and its atomic number is 25.Its abbreviated electronic configuration can be written as [Ar] 3d54s2.
When it loses three electrons it leads to the formation of Mn3+ cation, and its abbreviated electronic configuration is written as [Ar] 3d4.
The distribution of electrons in the 3d orbitals when no ligand is present is given as follows:
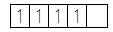
In case of octahedral complexes, the distribution of electrons in the five d-orbitals takes place according to the crystal field theory. According to this, in octahedral complexes as the ligand approaches the central metal atom, its d -orbitals get split into lower energy orbitals
Strong field ligands interact directly with the metal d-electrons and causes pairing of the electrons. The compound so formed is a low spin complex.
The distribution of electrons is shown below:
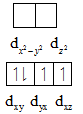
In case of low spin complexes, the unpaired electrons present in the higher energy gets paired with the electrons present in the t2g orbitals. From the above electronic distribution, Mn3+ does not contains two unpaired electrons.
(c)
Interpretation:
The number of unpaired electrons in octahedral complexes with strong field ligands for the metal ion
Concept introduction:
Coordination compounds are those in which a transition metal atom is bonded toa ligand which can be neutral, cation or anion. A transition metal cation has no outer s- electrons available for bonding, rather the inner d -electrons (in case of 3d transition metal elements) are available for making coordinate bonds with the ligands. Electrons are distributed in the five d- orbitals according to Hund’s rule which results in a maximum number of unpaired electrons. The abbreviated electronic configuration of an element depicts the electronic configuration of the elements by making use of noble gas configuration as they have fully-filled electron shells.
Answer to Problem 41QAP
There are zero unpaired electrons in Ag+.
Explanation of Solution
Silver is a 4d transition metal element and its atomic number is 47.Its abbreviated electronic configuration can be written as [Kr] 4d10 5s1.
When it losesone electron it leads to the formation of Ag+ cation, and its abbreviated electronic configuration is written as [Kr] 4d10.
The distribution of electrons in the 4d orbitals when no ligand is present is given as follows:

In case of octahedral complexes, the distribution of electrons in the five d-orbitals takes place according to the crystal field theory according to which, in octahedral complexes, as the ligand approaches the central metal atom, its d -orbitals get split into lower energy orbitals
Strong field ligands interact directly with the metal d-electrons and causes pairing of the electrons. The compound so formed is a low spin complex.
The distribution of electrons is shown below:
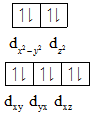
The electrons remain paired in case of strong filed ligands also. From the above electronic distribution, Ag+ does not contain any unpaired electrons.
(d)
Interpretation:
The number of unpaired electrons in octahedral complexes with strong field ligands for the metal ion
Concept introduction:
Coordination compounds are those in which a transition metal atom is bonded toa ligand which can be neutral, cation or anion. A transition metal cation has no outer s- electrons available for bonding, rather the inner d -electrons (in case of 3d transition metal elements) are available for making coordinate bonds with the ligands. Electrons are distributed in the five d- orbitals according to Hund’s rule which results in a maximum number of unpaired electrons. The abbreviated electronic configuration of an element depicts the electronic configuration of the elements by making use of noble gas configuration as they have fully-filled electron shells.
Answer to Problem 41QAP
There are zero unpaired electrons in Pt4+.
Explanation of Solution
In case of transition metal cations, the electrons that are present beyond the noble gas are located in their inner d- orbitals (5d orbitals in case of 5d transition metal elements), this means that they have no outer s- electrons and the distribution of electrons is according to Hund’s rule which states that when orbitals of equal energy are available, then electrons enter singly in the respective orbitals, this gives rise to maximum number of unpaired electrons in transition metal cations.
Platinum is a 5d transition metal element and its atomic number is 78.Its abbreviated electronic configuration can be written as [Xe] 4f145d96s1.
When it losesfour electrons it leads to the formation of Pt4+ cation, and its abbreviated electronic configuration is written as [Xe] 4f14 5d6
The distribution of electrons in the 5d orbitals when no ligand is present is given as follows:

In case of octahedral complexes, the distribution of electrons in the five d-orbitals takes place according to the crystal field theory according to which in octahedral complexes as the ligand approaches the central metal atom its d -orbitals get split into lower energy orbitals
Strong field ligands interact directly with the metal d-electrons and causes pairing of the electrons. The compound so formed is a low spin complex.
The distribution of electrons is shown below:
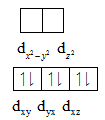
In case of low spin complexes the unpaired electrons present in the higher energy gets paired with the electrons present in the t2g orbitals. From the above electronic distribution, Pt4+ does not contain any unpaired electrons.
(e)
Interpretation:
The number of unpaired electrons in octahedral complexes with strong field ligands for the metal ion
Concept introduction:
Coordination compounds are those in which a transition metal atom is bonded toa ligand which can be neutral, cation or anion. A transition metal cation has no outer s- electrons available for bonding, rather the inner d -electrons (in case of 3d transition metal elements) are available for making coordinate bonds with the ligands. Electrons are distributed in the five d- orbitals according to Hund’s rule which results in a maximum number of unpaired electrons. The abbreviated electronic configuration of an element depicts the electronic configuration of the elements by making use of noble gas configuration as they have fully-filled electron shells.
Answer to Problem 41QAP
There are zero unpaired electrons in Au3+.
Explanation of Solution
Gold is a 5d transition metal element and its atomic number is 79. Its abbreviated electronic configuration can be written as [Xe] 4f14 5d10 6s1.
When it loses three electrons it leads to the formation of Au3+ cation, and its abbreviated electronic configuration is written as [Xe] 4f14 5d8
The distribution of electrons in the 5d orbitals when no ligand is present is given as follows:
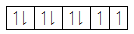
In case of octahedral complexes, the distribution of electrons in the five d-orbitals takes place according to the crystal field theory according to which in octahedral complexes as the ligand approaches the central metal atom its d -orbitals get split into lower energy orbitals
Strong field ligands interact directly with the metal d-electrons and causes pairing of the electrons. The compound so formed is a low spin complex.
The distribution of electrons is shown below:
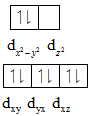
In case of low spin complexes the unpaired electron present in the higher energy
Want to see more full solutions like this?
Chapter 19 Solutions
PRINCIPLES+REACTIONS
- So, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forwardDraw a mental model for calcium chloride mixed with sodium phosphatearrow_forward
- here is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forwardDrawing of 3-fluro-2methylphenolarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





