
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 27P
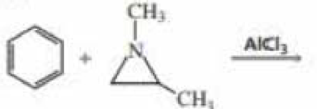
Benzene undergoes electrophilic aromatic substitution reactions with aziridines in the presence of a Lewis acid such as AlCl3.
- a. What are the major and minor products of the following reaction?
- b. Would you expect
epoxides to undergo similar reactions?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2CIO2 + 20H-1 CIO31 + CIO2 + H2O
Experiment
[CIO2], M
[OH-1], M
1
0.0500
0.100
23
2
0.100
0.100
3
0.100
0.0500
Initial Rate, M/s
0.0575
0.230
0.115
...
Given this date, calculate the overall order of this reaction.
2
3
.(be)_[Ɔ+(be)_OI ← (b²)_IƆO+ (be)_I
Experiment
[1-] M
0.005
[OCI-]
0.005
Initial Rate M/min
0.000275
0.0025
0.005
0.000138
0.0025
0.0025
0.000069
4
0.0025
0.0025
0.000140
Calculate the rate constant of this reaction using the table data.
1
2
3
4
I(aq) +OCl(aq) → IO¯¯(aq) + Cl¯(aq)
Experiment
[I-] M
0.005
[OCI-]
0.005
Initial Rate M/min
0.000275
0.0025
0.005
0.000138
0.0025
0.0025
Calculate the overall order of this reaction using the table data.
0.0025
0.000069
0.0025
0.000140
Chapter 19 Solutions
EBK ORGANIC CHEMISTRY
Ch. 19.1 - Name the following:Ch. 19.2 - Prob. 3PCh. 19.2 - Prob. 4PCh. 19.3 - Draw the product of each of the following...Ch. 19.5 - Prob. 6PCh. 19.5 - Explain why cyclopentadiene (pKa = 15) is more...Ch. 19.5 - When pyrrole is added to a dilute solution of...Ch. 19.6 - Prob. 10PCh. 19.6 - How to the mechanisms of the following reactions...Ch. 19.6 - Prob. 12P
Ch. 19.6 - Rank the following compounds from easiest to...Ch. 19.7 - Prob. 14PCh. 19.7 - Prob. 15PCh. 19.7 - Prob. 16PCh. 19.7 - Prob. 17PCh. 19.7 - Prob. 18PCh. 19.7 - Prob. 19PCh. 19.7 - Prob. 20PCh. 19 - Name the following:Ch. 19 - Prob. 22PCh. 19 - Rank the following compounds from strongest acid...Ch. 19 - Which of the following compounds is easier to...Ch. 19 - Rank the following compounds from most reactive to...Ch. 19 - One of the following compounds undergoes...Ch. 19 - Benzene undergoes electrophilic aromatic...Ch. 19 - Pyrrole reacts with excess...Ch. 19 - The dipole moments of furan and tetrahydrofuran...Ch. 19 - Name the following:Ch. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - a. Draw resonance contributors to show why...Ch. 19 - The chemical shifts of the C-2 hydrogen in the...Ch. 19 - Explain why protonating aniline has a dramatic...Ch. 19 - Prob. 36PCh. 19 - Propose a mechanism for the following reaction:Ch. 19 - Prob. 38PCh. 19 - Propose a mechanism for the following reactions:Ch. 19 - Prob. 40PCh. 19 - Prob. 41PCh. 19 - Prob. 42PCh. 19 - Organic chemists work with tetraphenylporphyrins...Ch. 19 - Show how the following compounds can be prepared...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give the IUPAC name for each compound.
Organic Chemistry
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal ...
Physics for Scientists and Engineers
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H2O2(aq) +3 I¯(aq) +2 H+(aq) → 13(aq) +2 H₂O(l)· ••• Experiment [H2 O2]o (M) [I]o (M) [H+]。 (M) Initial rate (M/s) 1 0.15 0.15 0.05 0.00012 234 0.15 0.3 0.05 0.00024 0.3 0.15 0.05 0.00024 0.15 0.15 0.1 0.00048 Calculate the overall order of this reaction using the table data.arrow_forwardThe U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m³ Part A If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 L.) ΜΕ ΑΣΦ = 2.35 1013 ? atoms ! Check your rounding. Your final answer should be rounded to 2 significant figures in the last step. No credit lost. Try again.arrow_forwardY= - 0.039 (14.01) + 0.7949arrow_forward
- Suppose 1.76 g of magnesium acetate (Mg (CH3CO2)2) are dissolved in 140. mL of water. Find the composition of the resulting electrolyte solution. In particular, list the chemical symbols (including any charge) of each dissolved ion in the table below. List only one ion per row. mEq Then, calculate the concentration of each ion in dwrite the concentration in the second column of each row. Be sure you round your answers to the L correct number of significant digits. ion Add Row mEq L x 5arrow_forwardA pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows.arrow_forwardNonearrow_forward
- Nonearrow_forwardDraw the structure of the product of the reaction given the IR and MS data. Spectral analysis of the product reveals: MS: M 150, M-15, M-43 CH.COCI AICI, IR: 3150-3000 cm, 2950-2850 cm and 1700 cmarrow_forwardPart II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic. a) HO b) Bri H HH c) d) H H H Br 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY