
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 25P
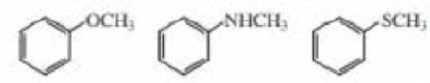
Rank the following compounds from most reactive to least reactive in an electrophilic aromatic substitution reaction:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Hi can you please help me solve these problems? thank you
Hi can you please help me solve these problems? thank you
Hi can you please help me solve this problem? thank you
Chapter 19 Solutions
EBK ORGANIC CHEMISTRY
Ch. 19.1 - Name the following:Ch. 19.2 - Prob. 3PCh. 19.2 - Prob. 4PCh. 19.3 - Draw the product of each of the following...Ch. 19.5 - Prob. 6PCh. 19.5 - Explain why cyclopentadiene (pKa = 15) is more...Ch. 19.5 - When pyrrole is added to a dilute solution of...Ch. 19.6 - Prob. 10PCh. 19.6 - How to the mechanisms of the following reactions...Ch. 19.6 - Prob. 12P
Ch. 19.6 - Rank the following compounds from easiest to...Ch. 19.7 - Prob. 14PCh. 19.7 - Prob. 15PCh. 19.7 - Prob. 16PCh. 19.7 - Prob. 17PCh. 19.7 - Prob. 18PCh. 19.7 - Prob. 19PCh. 19.7 - Prob. 20PCh. 19 - Name the following:Ch. 19 - Prob. 22PCh. 19 - Rank the following compounds from strongest acid...Ch. 19 - Which of the following compounds is easier to...Ch. 19 - Rank the following compounds from most reactive to...Ch. 19 - One of the following compounds undergoes...Ch. 19 - Benzene undergoes electrophilic aromatic...Ch. 19 - Pyrrole reacts with excess...Ch. 19 - The dipole moments of furan and tetrahydrofuran...Ch. 19 - Name the following:Ch. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - a. Draw resonance contributors to show why...Ch. 19 - The chemical shifts of the C-2 hydrogen in the...Ch. 19 - Explain why protonating aniline has a dramatic...Ch. 19 - Prob. 36PCh. 19 - Propose a mechanism for the following reaction:Ch. 19 - Prob. 38PCh. 19 - Propose a mechanism for the following reactions:Ch. 19 - Prob. 40PCh. 19 - Prob. 41PCh. 19 - Prob. 42PCh. 19 - Organic chemists work with tetraphenylporphyrins...Ch. 19 - Show how the following compounds can be prepared...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forwardTopic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTopic: Photochemistry and Photophysics of Supramoleculesarrow_forward
- How to name hydrocarbonsarrow_forwardPlease do these questions within the SCH4U course please with full steps since I am still unsure how to format my answers! Thank you so much.arrow_forwardWhen two solutions, one of 0.1 M KCl (I) and the other of 0.1 M MCl (II), are brought into contact by a membrane. The cation M cannot cross the membrane. At equilibrium, x moles of K+ will have passed from solution (I) to (II). To maintain the neutrality of the two solutions, x moles of Cl- will also have to pass from I to II. Explain this equality: (0.1 - x)/x = (0.1 + x)/(0.1 - x)arrow_forward
- Calculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.arrow_forwardGiven the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.arrow_forwardThe decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License