
EBK PHYSICS FOR SCIENTISTS AND ENGINEER
9th Edition
ISBN: 8220100581557
Author: Jewett
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 19.67AP
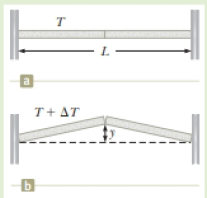
You are watching a new bridge being built near your house. You notice during the construction that two concrete spans of the bridge of total length Li = 250 m are placed end to end so that no room is allowed for expansion (Fig. P18.11a). In the opening storyline for this chapter, we talked about buckling sidewalks. The same thing will happen with spans on bridges if allowance is not made for expansion (Fig. P18.11b). You want to warn the construction crew about this dangerous situation, so you calculate the height y to which the spans will rise when they buckle in response to a temperature increase of ΔT = 20.0°C.
Figure P18.11
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please solve and answer all parts of the question correctly please. Thank you!!
Please solve and answer this question correctly please. Thank you!!
Please solve and answer the question correctly please. Thank you!!
Chapter 19 Solutions
EBK PHYSICS FOR SCIENTISTS AND ENGINEER
Ch. 19 - Prob. 19.1QQCh. 19 - Consider the following pairs of materials. Which...Ch. 19 - If you are asked to make a very sensitive glass...Ch. 19 - Two spheres are made of the same metal and have...Ch. 19 - A common material for cushioning objects in...Ch. 19 - On a winter day, you turn on your furnace and the...Ch. 19 - Markings to indicate length are placed on a steel...Ch. 19 - When a certain gas under a pressure of 5.00 106...Ch. 19 - If the volume of an ideal gas is doubled while its...Ch. 19 - The pendulum of a certain pendulum clock is made...
Ch. 19 - A temperature of 162F is equivalent to what...Ch. 19 - A cylinder with a piston holds 0.50 m3 of oxygen...Ch. 19 - What would happen if the glass of a thermometer...Ch. 19 - A cylinder with a piston contains a sample of a...Ch. 19 - Two cylinders A and B at the same temperature...Ch. 19 - A rubber balloon is filled with 1 L of air at 1...Ch. 19 - The average coefficient of linear expansion of...Ch. 19 - Suppose you empty a tray of ice cubes into a bowl...Ch. 19 - A hole is drilled in a metal plate. When the metal...Ch. 19 - On a very cold day in upstate New York, the...Ch. 19 - Common thermometers are made of a mercury column...Ch. 19 - A piece of copper is dropped into a beaker of...Ch. 19 - Prob. 19.3CQCh. 19 - Some picnickers stop at a convenience store to buy...Ch. 19 - Prob. 19.5CQCh. 19 - Prob. 19.6CQCh. 19 - An automobile radiator is filled to the brim with...Ch. 19 - When the metal ring and metal sphere in Figure...Ch. 19 - Prob. 19.9CQCh. 19 - Prob. 19.10CQCh. 19 - Prob. 19.1PCh. 19 - The temperature difference between the inside and...Ch. 19 - Prob. 19.3PCh. 19 - Prob. 19.4PCh. 19 - Liquid nitrogen has a boiling point of 195.81C at...Ch. 19 - Death Valley holds the record for the highest...Ch. 19 - Prob. 19.7PCh. 19 - The concrete sections of a certain superhighway...Ch. 19 - The active element of a certain laser is made of a...Ch. 19 - Prob. 19.10PCh. 19 - A copper telephone wire has essentially no sag...Ch. 19 - A pair of eyeglass frames is made of epoxy...Ch. 19 - The Trans-Alaska pipeline is 1 300 km long,...Ch. 19 - Prob. 19.14PCh. 19 - A square hole 8.00 cm along each side is cut in a...Ch. 19 - The average coefficient of volume expansion for...Ch. 19 - At 20.0C, an aluminum ring has an inner diameter...Ch. 19 - Why is the following situation impossible? A thin...Ch. 19 - A volumetric flask made of Pyrex is calibrated at...Ch. 19 - Review. On a day that the temperature is 20.0C, a...Ch. 19 - Prob. 19.21PCh. 19 - Review. The Golden Gate Bridge in San Francisco...Ch. 19 - Prob. 19.23PCh. 19 - A sample of a solid substance has a mass m and a...Ch. 19 - An underground gasoline lank can hold 1.00 103...Ch. 19 - A rigid lank contains 1.50 moles of an ideal gas....Ch. 19 - Prob. 19.27PCh. 19 - Your father and your younger brother are...Ch. 19 - Gas is contained in an 8.00-L vessel al a...Ch. 19 - A container in the shape of a cube 10.0 cm on each...Ch. 19 - An auditorium has dimensions 10.0 m 20.0 m 30.0...Ch. 19 - The pressure gauge on a lank registers the gauge...Ch. 19 - Prob. 19.33PCh. 19 - Prob. 19.34PCh. 19 - A popular brand of cola contains 6.50 g of carbon...Ch. 19 - In state-of-the-art vacuum systems, pressures as...Ch. 19 - An automobile tire is inflated with air originally...Ch. 19 - Review. To measure how far below the ocean surface...Ch. 19 - Review. The mass of a hot-air balloon and its...Ch. 19 - A room of volume V contains air having equivalent...Ch. 19 - Review. At 25.0 in below the surface of the sea,...Ch. 19 - Prob. 19.42PCh. 19 - A cook puts 9.00 g of water in a 2.00-L pressure...Ch. 19 - The pressure gauge on a cylinder of gas registers...Ch. 19 - Prob. 19.45APCh. 19 - A steel beam being used in the construction of a...Ch. 19 - A spherical steel ball bearing has a diameter of...Ch. 19 - A bicycle tire is inflated to a gauge pressure of...Ch. 19 - In a chemical processing plant, a reaction chamber...Ch. 19 - Why is the following situation impossible? An...Ch. 19 - A mercury thermometer is constructed as shown in...Ch. 19 - A liquid with a coefficient of volume expansion ...Ch. 19 - Prob. 19.53APCh. 19 - Two metal bars are made of invar and a third bar...Ch. 19 - A student measures the length of a brass rod with...Ch. 19 - The density of gasoline is 730 kg/m3 at 0C. Its...Ch. 19 - A liquid has a density . (a) Show that the...Ch. 19 - Prob. 19.58APCh. 19 - Review. A dock with a brass pendulum has a period...Ch. 19 - A bimetallic strip of length L is made of two...Ch. 19 - The rectangular plate shown in Figure P18.37 has...Ch. 19 - The measurement of the average coefficient of...Ch. 19 - Prob. 19.63APCh. 19 - A vertical cylinder of cross-sectional area A is...Ch. 19 - Review. Consider an object with any one of the...Ch. 19 - (a) Show that the density of an ideal gas...Ch. 19 - You are watching a new bridge being built near...Ch. 19 - You are watching a new bridge being built near...Ch. 19 - Review. (a) Derive an expression for the buoyant...Ch. 19 - Prob. 19.70APCh. 19 - Starting with Equation 18.11, show that the total...Ch. 19 - Review. A steel wire and a copper wire, each of...Ch. 19 - Review. A steel guitar string with a diameter of...Ch. 19 - A cylinder is closed by a piston connected to a...Ch. 19 - Prob. 19.75CPCh. 19 - A cylinder that has a 40.0-cm radius and is 50.0...Ch. 19 - Prob. 19.77CPCh. 19 - Review. A house roof is a perfectly flat plane...Ch. 19 - A 1.00-km steel railroad rail is fastened securely...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- In a scene from The Avengers (the first one) Black Widow is boosted directly upwards by Captain America, where she then grabs on to a Chitauri speeder that is 15.0 feet above her and hangs on. She is in the air for 1.04 s. A) With what initial velocity was Black Widow launched? 1 m = 3.28 ft B) What was Black Widow’s velocity just before she grabbed the speeder? Assume upwards is the positive direction.arrow_forwardIn Dark Souls 3 you can kill the Ancient Wyvern by dropping on its head from above it. Let’s say you jump off the ledge with an initial velocity of 3.86 mph and spend 1.72 s in the air before hitting the wyvern’s head. Assume the gravity is the same as that of Earth and upwards is the positive direction. Also, 1 mile = 1609 m. A) How high up is the the ledge you jumped from as measured from the wyvern’s head? B) What is your velocity when you hit the wyvern?arrow_forwardA) If Yoshi flings himself downwards at 9.76 miles per hour to hit an enemy 10.5 m below him, how fast is Yoshi traveling when he hits the enemy? 1 mile = 1609 marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

A Level Physics – Ideal Gas Equation; Author: Atomi;https://www.youtube.com/watch?v=k0EFrmah7h0;License: Standard YouTube License, CC-BY