
Concept explainers
(a)
Interpretation:
The type of interaction present between the side chains of tyrosine and glutamine is to be stated.
Concept introduction:
The structure of proteins has four stages. These four stages are: primary, secondary, tertiary and quaternary structures. In primary structure, the amino acids are arranged to give the backbone of protein. In secondary structure, the pattern of the peptide chain is arranged in certain order. In tertiary structure, the interactions between the side chains of amino acids are considered. In quaternary structure, two or more peptide chains are linked.
Answer to Problem 19.42E
The type of interaction present between the side chains of tyrosine and glutamine is hydrogen bonding.
Explanation of Solution
The structures of tyrosine and glutamine are shown in Figure 1.
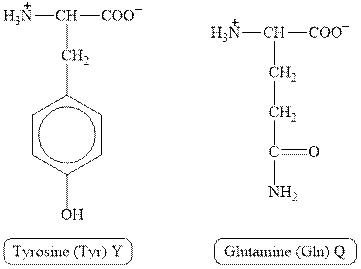
Figure 1
In tyrosine, the side chain has
Therefore, the type of interaction present between the side chains of tyrosine and glutamine is hydrogen bonding.
The type of interaction present between the side chains of tyrosine and glutamine is hydrogen bonding.
(b)
Interpretation:
The type of interaction present between the side chains of aspartate and lysine is to be stated.
Concept introduction:
The structure of proteins has four stages. These four stages are: primary, secondary, tertiary and quaternary structures. In primary structure, the amino acids are arranged to give the backbone of protein. In secondary structure, the pattern of the peptide chain is arranged in certain order. In tertiary structure, the interactions between the side chains of amino acids are considered. In quaternary structure, two or more peptide chains are linked.
Answer to Problem 19.42E
The type of interaction present between the side chains of aspartate and lysine is salt bridge.
Explanation of Solution
The structures of aspartate and lysine are shown in Figure 2.
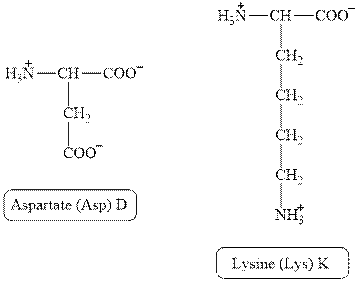
Figure 2
In aspartate, the side chain has
The type of interaction present between the side chains of aspartate and lysine is salt bridge.
(c)
Interpretation:
The type of interaction present between the side chains of leucine and isoleucine is to be stated.
Concept introduction:
The structure of proteins has four stages. These four stages are: primary, secondary, tertiary and quaternary structures. In primary structure, the amino acids are arranged to give the backbone of protein. In secondary structure, the pattern of the peptide chain is arranged in certain order. In tertiary structure, the interactions between the side chains of amino acids are considered. In quaternary structure, two or more peptide chains are linked.
Answer to Problem 19.42E
The type of interaction present between the side chains of leucine and isoleucine is hydrophobic interaction.
Explanation of Solution
The structures of leucine and isoleucine are shown in Figure 3.
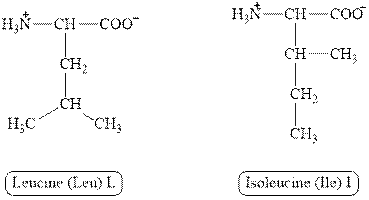
Figure 3
In leucine, the side chain has
The type of interaction present between the side chains of leucine and isoleucine is hydrophobic interaction.
(d)
Interpretation:
The type of interaction present between the side chains of phenylalanine and valine is to be stated.
Concept introduction:
The structure of proteins has four stages. These four stages are: primary, secondary, tertiary and quaternary structures. In primary structure, the amino acids are arranged to give the backbone of protein. In secondary structure, the pattern of the peptide chain is arranged in certain order. In tertiary structure, the interactions between the side chains of amino acids are considered. In quaternary structure, two or more peptide chains are linked.
Answer to Problem 19.42E
The type of interaction present between the side chains of phenylalanine and valine is hydrophobic interaction.
Explanation of Solution
The structures of phenylalanine and valine are shown in Figure 4.
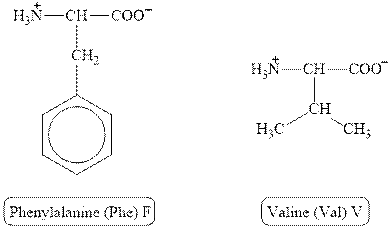
Figure 4
In phenylalanine, the side chain has
The type of interaction present between the side chains of phenylalanine and valine is hydrophobic interaction.
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry for Today: General Organic and Biochemistry
- What is the mechanism for this?arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward7. Describe the expected 31P and 19F (where applicable) NMR spectral patterns for the following compounds (indicate number of signals and their splitting patterns). a) tetraphenyldiphosphine Ph Ph P-P Ph Ph Ph Ph ' b) tetraphenyldiphosphine monoxide P-P-Ph Ph (2 points) (2 points c) tetrafluorophosphonium hexafluorophosphate [PF4]*[PF6]¯ (4 points)arrow_forward
- 3. For questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ Which (if any) of these complexes would be expected to display Jahn-Teller distortion? (2 points)arrow_forwardWhat is Instrumental Neutron Activation and what are the advantages and disadvantages in using its applications? (I'm doing an in class assignment and need better understanding of what the instrument can be used for) Please include references so that I can better understand the application of how the instrument works!arrow_forwardWhat is Isotope Analysis and what are the advantages and disadvantages in using its applications and instrumentalization? Please include references so that I can better understand how the instrument works!arrow_forward
- 5. Count the electrons on the following complexes and state whether they follow the 18- electron rule: (3 points) Fe(CO)5 Ni(PMe3)4 PMe3 is trimethylphosphine Mn(CO)5Brarrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6]+, [CoCl4]², [Cr(H2O)6]²+ 2. Draw the corresponding d-orbital splitting for each of the complexes; predict the spin- state (low-spin/high spin) for each of the complexes (if applicable); explain your arguments. Calculate the crystal field stabilization energy for each complex (in Ao or At). (6 points)arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ 1. Assign oxidation number to the metal, then indicate d-electron count. (3 points)arrow_forward
- Using iodometry I want to titrate a sodium thiosulfate solution and I use 15 mL. If I have 50 mL of a 0.90 M copper solution and KI, what will be the molarity of sodium thiosulfate?arrow_forwardDraw the product formed when the following pair of compounds is treated with NaOEt in ethanol. + i CNarrow_forwardI need help with the followingarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





