
Concept explainers
Identify the
a. tyrosine (neutral, polar)
b. glutamate (acidic, polar)
c. methionine (neutral, nonpolar)
d. histidine (basic, polar)
e. cysteine (neutral, polar)
f. valine (neutral, nonpolar)
(a)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
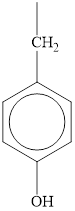
The structure of tyrosine is given below.
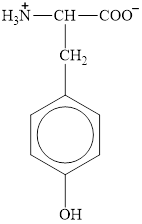
Figure 1
Therefore, the

Figure 2
The
(b)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
![]()
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
The structure of glutamate is given below.
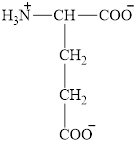
Figure 3
Therefore, the
![]()
Figure 4
The
(c)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
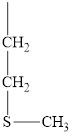
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
The structure of methionine is given below.
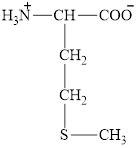
Figure 5
Therefore, the
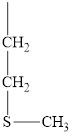
Figure 6
The
(d)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
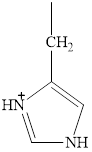
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
The structure of histidine is given below.
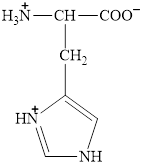
Figure 7
Therefore, the

Figure 8
The
(e)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
![]()
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
The structure of cysteine is given below.
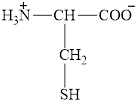
Figure 9
Therefore, the
![]()
Figure 10
The
(f)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
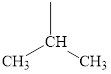
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
The structure of valine is given below.
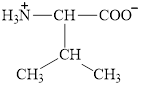
Figure 11
Therefore, the
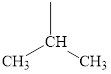
Figure 12
The
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry for Today: General Organic and Biochemistry
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





