
(a)
Interpretation:
The compound should be identified using the following proton NMR spectra whose empirical formula is C8H10:
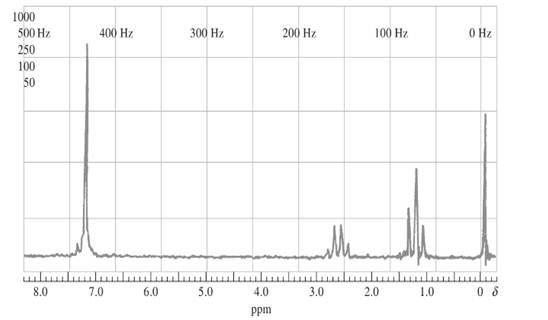
Concept introduction:
Proton NMR is application of nuclear magnetic resonance with respect to 1H nuclei in molecules in order to determine structure. Protons that are in the same magnetic environment are considered as chemically equivalent protons.
(b)
Interpretation:
The compound should be identified using the following proton NMR spectra whose empirical formula is C8H10:
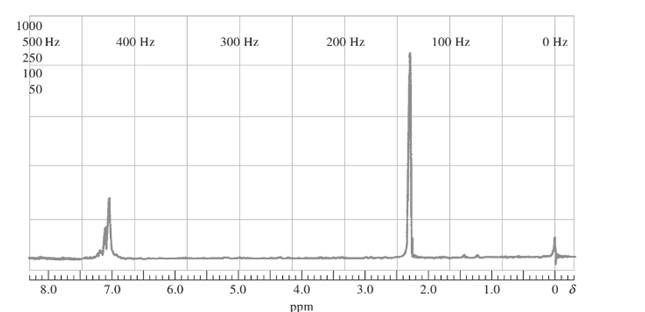
Concept introduction:
Proton NMR is application of nuclear magnetic resonance with respect to 1H nuclei in molecules in order to determine structure. Protons that are in the same magnetic environment are considered as chemically equivalent protons.
Trending nowThis is a popular solution!

Chapter 19 Solutions
Principles of Instrumental Analysis, 6th Edition
- Draw the mechanism for the substitution reaction converting an alcohol into an alkyl halide. If chirality is important to the reaction include it.arrow_forwardWrite, in words three different reactions we can use to make an alcohol.arrow_forwardDraw the reduction mechanism for the reduction of the aldehyde.arrow_forward
- What is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forwardWhile noble gas exerts the strongest London (dispersion) forces on neighboring atoms? Group of answer choices Xe Ar Kr Nearrow_forwardWhich of the following elements is corrosive to your skin due to that element breaking down C=C bonds? Group of answer choices fluorine iodine bromine chlorinearrow_forward
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


