
Concept explainers
(a)
Interpretation: The formula of rutile as TiO2 on the basis of given unit cell needs to be determined.
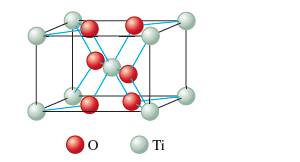
Concept Introduction:
Each solid has a different type of unit cell and crystal lattice that makes it unique from other solids. The atoms are arranged in unit cells.
The unit cells in the solids can arrange in different ways. In a crystal each particle can be represented as spherical atoms which are packed together and bonded to each other equally in all directions. It is called closest packing of crystal that can be in hexagonal cubic packing, close cubic packing, face center cubic packing, etc.
(b)
Interpretation: The oxidation number, for both reactions with identification of oxidizing and reducing agent for each reaction needs to be done.
Concept Introduction:
Each solid has a different type of unit cell and crystal lattice that makes it unique from other solids. The atoms are arranged in unit cells.
The unit cells in the solids can arrange in different ways. In a crystal, each particle can be represented as spherical atoms which are packed together and bonded to each other equally in all directions. It is called closest packing of crystal that can be in hexagonal cubic packing, close cubic packing, face center cubic packing, etc.
Trending nowThis is a popular solution!

Chapter 19 Solutions
EBK WEBASSIGN FOR ZUMDAHL'S CHEMICAL PR
- 8.96 A business manager wants to provide a wider range of p- and n-type semiconductors as a strategy to enhance sales. You are the lead materials engineer assigned to communicate with this manager. How would you explain why there are more ways to build a p-type semiconductor from silicon than there are ways to build an n-type semiconductor from silicon?arrow_forwardWhy is the formation of slag useful during the smelting of iron?arrow_forwardThe precious metal platinum was first used by indigenous peoples of South America, who found impure, native samples of it in the Au mines of what is now Ecuador and used the samples to make small items of jewelry. Platinum’s high melting point (1772oC) makes it harder to work than Au (1064oC) and Ag (962oC), but this same property and a high resistance to chemical attack make Pt suitable as a material for high-temperature crucibles. Although Pt is a noble metal, in the +4 and +2 states it forms a variety of compounds, many of which are coordination complexes. Its coordinating abilities make it an important catalyst for organic and inorganic reactions. The anticancer cisplatin (mentioned during lectures) can be prepared from K2PtCl6 via reduction with hydrazine (N2H4), giving K2PtCl4, followed by replacement of the 2 Cl ion ligands with NH3. Give the names to the three platinum compounds referred to. Draw coordination complex trans-Platinum diammine dichloride and label the Pt with its…arrow_forward
- The precious metal platinum was first used by indigenous peoples of South America, who found impure, native samples of it in the Au mines of what is now Ecuador and used the samples to make small items of jewelry. Platinum’s high melting point (1772oC) makes it harder to work than Au (1064oC) and Ag (962oC), but this same property and a high resistance to chemical attack make Pt suitable as a material for high-temperature crucibles. Although Pt is a noble metal, in the +4 and +2 states it forms a variety of compounds, many of which are coordination complexes. Its coordinating abilities make it an important catalyst for organic and inorganic reactions. Draw coordination complex trans-Platinum diammine dichloride and label the Pt with its oxidation number. For Pt4+, draw energy level diagrams of the d-AOs and show orbital occupancy in both weak and strong octahedral fields. Calculate the total spin for each case. Calculate the CFSE (crystal field stabilization energy) in terms of Δ0 for…arrow_forward13. Show that the maximum packing ratio in the diamond structure is n/3/16. [Hint: The structure may be viewed as two interpenetrating fcc lattices, arranged such that each atom is surrounded by four other atoms, forming a regular tetrahedron.] 14. A quantitative theory of bonding in ionic crystals was developed by Born and Meyer along the following lines: The total potential energy of the system is taken to be ae? E = N-arrow_forwardA unit cell of Re03 consists of a cubic .1 arrangement of Re atoms with O atoms centred along each edge of the cube. The coordination number of each Re atom isarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





