
ORG CHEM CONNECT CARD
6th Edition
ISBN: 9781264860746
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18.2, Problem 3P
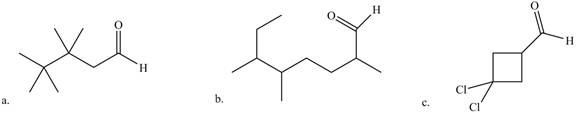
Give the IUPAC name for each
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
+ HCl →?
Draw the molecule on the canvas by choosing buttons from the Tools (for bonas), Atoms
and Advanced Template toolbars. The single bond is active by default.
+
M
C
+
H± 2D
EXP. CONT. K
?
L
1
H₁₂C
[1]
A
HCN O
S
CH3
CH 3
CI
Br
HC
H₂
CH
CH
CH3
-
P
F
SH
SH
0
Chapter 18 Solutions
ORG CHEM CONNECT CARD
Ch. 18.1 - Rank the following compounds in order of...Ch. 18.1 - Prob. 2PCh. 18.2 - Give the IUPAC name for each aldehyde.Ch. 18.2 - Prob. 4PCh. 18.2 - Give the IUPAC name for each ketone.Ch. 18.5 - Prob. 11PCh. 18.9 - Problem 21.17 Draw the products of the following...Ch. 18.9 - Problem 21.18 Outline a synthesis of each Wittig...Ch. 18.9 - Problem 21.19 Draw the products (including...Ch. 18.9 - Problem 21.20 What starting materials are needed...
Ch. 18.9 - Prob. 19PCh. 18.10 - Problem 21.22 The product formed when reacts with...Ch. 18.10 - Prob. 21PCh. 18.11 - Prob. 22PCh. 18.11 - Prob. 23PCh. 18.11 - Prob. 24PCh. 18.12 - Prob. 25PCh. 18.12 - Problem 21.28 Draw a stepwise mechanism for the...Ch. 18.13 - Problem 21.29 Draw the products of each...Ch. 18 - Problem 21.40 (a) Give the IUPAC name for A and B....Ch. 18 - 21.41 Rank the following compounds in order of...Ch. 18 - Prob. 39PCh. 18 - 21.43 Give the IUPAC name for each compound.
a....Ch. 18 - 21.44 Give the structure corresponding to each...Ch. 18 - Prob. 42PCh. 18 - 21.46 Draw the products of each reaction.
a. e....Ch. 18 - Prob. 44PCh. 18 - 21.48 Draw all stereoisomers formed in each...Ch. 18 - Prob. 54PCh. 18 - Prob. 55PCh. 18 - Prob. 56PCh. 18 - Devise a synthesis of each alkene using a Wittig...Ch. 18 - Prob. 60PCh. 18 - Prob. 62PCh. 18 - Prob. 63PCh. 18 - 21.64 Draw a stepwise mechanism for the following...Ch. 18 - 21.65 Draw a stepwise mechanism f or the following...Ch. 18 - Prob. 67PCh. 18 - 21.67 Draw a stepwise mechanism for each...Ch. 18 - Prob. 69PCh. 18 - Prob. 70P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Please consider the two all 'cis' isomers of trimethylcyclohexane drawn below. Draw the two chair conformers of each stereoisomer below (1 and 2) and calculate their torsional interaction energies in order to identify the lower energy conformer for each stereoisomer. Based on your calculations, state which of the two stereoisomers 1 and 2 is less stable and which is more stable. [1,3-diaxial CH3 CH3 = 3.7kcal/mol; 1,3-diaxial CH3 H = 0.88kcal/mol; cis-1,2 (axial:equatorial) CH3 CH3 = 0.88kcal/mol; trans-1,2-diequatorial CH3 CH3 = 0.88kcal/mol) all-cis-1,2,3- 1 all-cis-1,2,4- 2arrow_forwardNonearrow_forwardWhat is the mechanism by which the 1,4 product is created? Please draw it by hand with arrows and stuff.arrow_forward
- What is the relationship between A and B? H3C A Br Cl H3C B Br relationship (check all that apply) O same molecule O enantiomer O diastereomer structural isomer O stereoisomer isomer O need more information to decide O same molecule ☐ enantiomer Br Br Br CH3 Br CI CH3 O diastereomer ☐ structural isomer ☐ stereoisomer isomer O need more information to decide O same molecule O enantiomer Odiastereomer structural isomer O stereoisomer ☐ isomer O need more information to decidearrow_forwardb. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 4arrow_forwardc. Serricornin, the female-produced sex pheromone of the cigarette beetle, has the following structure. OH What is the maximum number of possible stereoisomers? Is this structure a meso compound? d. Please consider the natural product alkaloids shown below. Are these two structures enantiomers, diastereomers or conformers? H HO H H HN HO HN R R с R=H cinchonidine R=ET cinchonine Harrow_forward
- Nail polish remover containing acetone was spilled in a room 5.23 m × 3.28 m × 2.76 m. Measurements indicated that 2,250 mg of acetone evaporated. Calculate the acetone concentration in micrograms per cubic meter.arrow_forwardPlease help me answer number 1. 1. If your graphs revealed a mathematical relationship between specific heat and atomic mass, write down an equation for the relationship. I also don't understand, is the equation from the line regression the one that I'm suppose use to show the relationship? If so could you work it all the way out?arrow_forwardDescribe the principle of resonance and give a set of Lewis Structures to illustrate your explanation.arrow_forward
- Don't used hand raitingarrow_forwardIt is not unexpected that the methoxyl substituent on a cyclohexane ring prefers to adopt the equatorial conformation. OMe H A G₂ = +0.6 kcal/mol OMe What is unexpected is that the closely related 2-methoxytetrahydropyran prefers the axial conformation: H H OMe OMe A Gp=-0.6 kcal/mol Methoxy: CH3O group Please be specific and clearly write the reason why this is observed. This effect that provides stabilization of the axial OCH 3 group in this molecule is called the anomeric effect. [Recall in the way of example, the staggered conformer of ethane is more stable than eclipsed owing to bonding MO interacting with anti-bonding MO...]arrow_forward206 Pb 82 Express your answers as integers. Enter your answers separated by a comma. ▸ View Available Hint(s) VAΣ ΜΕ ΑΣΦ Np, N₁ = 82,126 Submit Previous Answers ? protons, neutronsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY