
PHYS 212 FOR SCI+ENG W/MAST PHYS >ICP<
1st Edition
ISBN: 9781323834831
Author: Knight
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 56EAP
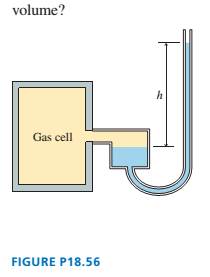
The mercury manometer shown in FIGURE P18.56 is attached
to a gas cell. The mercury height h is 120 mm when the cell is
placed in an ice-water mixture. The mercury height drops to
30 mm when the device is carried into an industrial freezer.
What is the freezer temperature?
Hint: The right tube of the manometer is much narrower than the
left tube. What reasonable assumption can you make about the gas
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
No chatgpt pls will upvote
You are working with a team that is designing a new roller coaster-type amusement park ride for a major theme park. You are present for the testing of the ride, in which an empty 150 kg car is sent along the entire ride. Near the end of the ride, the car is at near rest at the top of a 100 m
tall track. It then enters a final section, rolling down an undulating hill to ground level. The total length of track for this final section from the top to the ground is 250 m. For the first 230 m, a constant friction force of 370 N acts from computer-controlled brakes. For the last 20 m, which is
horizontal at ground level, the computer increases the friction force to a value required for the speed to be reduced to zero just as the car arrives at the point on the track at which the passengers exit.
(a) Determine the required constant friction force (in N) for the last 20 m for the empty test car.
Write AK + AU + AE int
= W+Q + TMW
+
TMT + TET + TER for the car-track-Earth system and solve for…
=
12 kg, and m3
Three objects with masses m₁ = 3.8 kg, m₂
find the speed of m3 after it moves down 4.0 m.
m/s
19 kg, respectively, are attached by strings over frictionless pulleys as indicated in the figure below. The horizontal surface exerts a force of friction of 30 N on m2. If the system is released from rest, use energy concepts to
m
m2
m3
i
Chapter 18 Solutions
PHYS 212 FOR SCI+ENG W/MAST PHYS >ICP<
Ch. 18 - Prob. 1CQCh. 18 - Prob. 2CQCh. 18 - Prob. 3CQCh. 18 - Prob. 4CQCh. 18 - Prob. 5CQCh. 18 - Prob. 6CQCh. 18 - Prob. 7CQCh. 18 - Prob. 8CQCh. 18 - Prob. 9CQCh. 18 - A gas undergoes the process shown in FIGURE...
Ch. 18 - Prob. 11CQCh. 18 - Prob. 12CQCh. 18 - Prob. 1EAPCh. 18 - Prob. 2EAPCh. 18 - What is the diameter of a copper sphere that has...Ch. 18 - Prob. 4EAPCh. 18 - Prob. 5EAPCh. 18 - How many atoms are in a 2.0 cm × 2.0 cm × 2.0 cm...Ch. 18 - Prob. 7EAPCh. 18 - An element in its solid phase has mass density...Ch. 18 - .0 mol of gold is shaped into a sphere. What is...Ch. 18 - What volume of aluminum has the same number of...Ch. 18 - Prob. 11EAPCh. 18 - Prob. 12EAPCh. 18 - Prob. 13EAPCh. 18 - A concrete bridge is built of 325-cm-long concrete...Ch. 18 - A surveyor has a steel measuring tape that is...Ch. 18 - Two students each build a piece of scientific...Ch. 18 - Prob. 17EAPCh. 18 -
18. What is the temperature in °F and the...Ch. 18 - Prob. 19EAPCh. 18 - .0 mol of gas at a temperature of -120°C fills a...Ch. 18 - Prob. 21EAPCh. 18 - Prob. 22EAPCh. 18 - Prob. 23EAPCh. 18 - Prob. 24EAPCh. 18 - Prob. 25EAPCh. 18 - Prob. 26EAPCh. 18 - Prob. 27EAPCh. 18 - Prob. 28EAPCh. 18 - A rigid, hollow sphere is submerged in boiling...Ch. 18 -
30. A rigid container holds hydrogen gas at a...Ch. 18 - Prob. 31EAPCh. 18 - Prob. 32EAPCh. 18 - Prob. 33EAPCh. 18 - Prob. 34EAPCh. 18 - Prob. 35EAPCh. 18 - Prob. 36EAPCh. 18 - Prob. 37EAPCh. 18 - .0050 mol of gas undergoes the process 1 2 3...Ch. 18 - Prob. 39EAPCh. 18 - Prob. 40EAPCh. 18 - Prob. 41EAPCh. 18 - Prob. 42EAPCh. 18 - Prob. 43EAPCh. 18 - A 15°C, 2.0-cm-diameter aluminum bar just barely...Ch. 18 - Prob. 45EAPCh. 18 - Prob. 46EAPCh. 18 - Prob. 47EAPCh. 18 - Prob. 48EAPCh. 18 - Prob. 49EAPCh. 18 - The 3.0-m-long pipe in FIGURE P18.50 is closed at...Ch. 18 - Prob. 51EAPCh. 18 - An electric generating plant boils water to...Ch. 18 - Prob. 53EAPCh. 18 - The air temperature and pressure in a laboratory...Ch. 18 - Prob. 55EAPCh. 18 - The mercury manometer shown in FIGURE P18.56 is...Ch. 18 - Prob. 57EAPCh. 18 - The 50 kg circular piston shown in FIGURE P18.58...Ch. 18 - Prob. 59EAPCh. 18 - .0 g of helium gas follows the process 1? 2 ?3...Ch. 18 - Prob. 61EAPCh. 18 - 62. FIGURE P18.62 shows two different processes...Ch. 18 - Prob. 63EAPCh. 18 - Prob. 64EAPCh. 18 - Prob. 65EAPCh. 18 - Prob. 66EAPCh. 18 - Prob. 67EAPCh. 18 - Prob. 68EAPCh. 18 - Prob. 69EAPCh. 18 - Prob. 70EAPCh. 18 - Prob. 71EAPCh. 18 - The cylinder in FIGURE CP18.72 has a moveable...Ch. 18 - Containers A and B in FIGURE CP18.73 hold the same...Ch. 18 - Prob. 74EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Three objects with masses m₁ = 3.8 kg, m₂ = 12 kg, and m 19 kg, respectively, are attached by strings over frictionless pulleys as indicated in the figure below. The horizontal surface exerts a force of friction of 30 N on m2. If the system is released from rest, use energy concepts to find the speed of m¸ after it moves down 4.0 m. m/s m m2 mgarrow_forwardIn order for Jane to return to base camp, she needs to swing across a river of width D that is filled with alligators. She must swing into a wind exerting constant horizontal force F, F = 110 N, L = 40.0 m, 0 = 50.0°, and her mass to be 50.0 kg. Wind →F Tarzan! Jane (a) with what minimum speed (in m/s) must Jane begin her swing to just make it to the other side? (If Jane can make it across with zero initial velocity, enter 0.) m/s on a vine having length L and initially making an angle with the vertical (see below figure). Take D = 48.0 m, (b) Shortly after Jane's arrival, Tarzan and Jane decide to swing back across the river (simultaneously). With what minimum speed (in m/s) must they begin their swing? Assume that Tarzan has a mass of 80.0 kg. m/sarrow_forwardR=2.00 12V 2.00 4.00 4.002 What is the current in one of the 4.0 Q resistors? An isolated point charge q is located at point X. Two other points Y and Z are such that YZ2 XY. Y X What is (electric field at Y)/(electric field at Z)?arrow_forward
- Two objects (m₁ = 4.75 kg and m₂ 2.80 kg) are connected by a light string passing over a light, frictionless pulley as in the figure below. The 4.75-kg object is released from rest at a point h = 4.00 m above the table mg m (a) Determine the speed of each object when the two pass each other. m/s (b) Determine the speed of each object at the moment the 4.75-kg object hits the table. m/s (c) How much higher does the 2.80-kg object travel after the 4.75-kg object hits the table? marrow_forwardA cell of negligible internal resistance is connected to three identical resistors. The current in the cell is 3.0 A. The resistors are now arranged in series. What is the new current in the cell?arrow_forwardA negatively charged sphere is falling through a magnetic field. north pole of magnet direction of motion south pole of magnet What is the direction of the magnetic force acting on the sphere?arrow_forward
- Electrons in a conductor are moving down the page. A proton outside the wire is moving to the right. What is the direction of the magnetic force acting on the proton?arrow_forwardWhat is the resistance of an ideal voltmeter and the resistance of an ideal ammeter? Resistance of an ideal voltmeter Resistance of an ideal ammeter infinite A. zero B. zero zero C. infinite infinite D. infinite zeroarrow_forwardvariable resistor with a resistance range of 0 to 6.0 KQ is connected in series with two resistors of fixed value 6.0 KQ. The cell in the circuit has an emf of 18 V and a negligible internal resistance. 18 V X Y 6.0 ΚΩ 6.0 ΚΩ 0 - 6.0 ΚΩ What is the maximum range of potential difference that can be observed between X and Y?arrow_forward
- A positive point charge of magnitude 1.0 μC and a point charge q are separated by a distance d. electron 1.0 με An electron is placed at a distance d from the +1.0 μC charge. The electric force on the electron is zero. What is q?arrow_forwardTwo point charges of +4q and -q are placed a fixed distance apart. Where is the electric field strength equal to zero? B. +49 D. A network of three resistors is connected to a cell of emf 12V and internal resistance R of 2.0 Q as shown.arrow_forwardThree point charges of equal magnitude are placed at the vertices of an equilateral triangle. The signs of the charges are shown. Point P is equidistant from the vertices of the triangle. What is the direction of the resultant electric field at P? B.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY