
Interpretation:
The short sequences of reactions that would be appropriate for the given transformations, using the indicated starting material are to be written.
Concept introduction:
Friedel Craft acylation is an electrophilic
Grignard reaction is a reaction in which aryl or
The molecule,
Dess Martin periodinane (DMP) is a reagent which is used for the oxidation of primary alcohols to
The Diels–Alder reaction is a
Answer to Problem 42P
Solution:
a) Short sequences of reactions that are appropriate for the transformation of
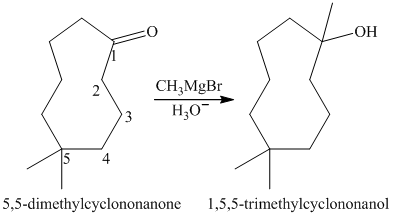
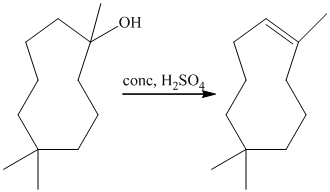
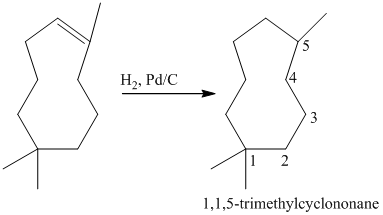
b) Short sequences of reactions for the transformation of the given compounds are shown below.
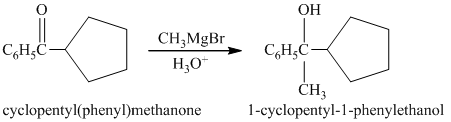
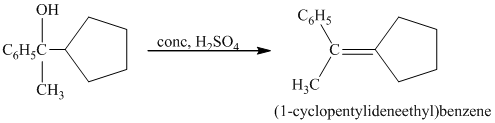
c) Short sequences of reactions for the transformation of the given compound from
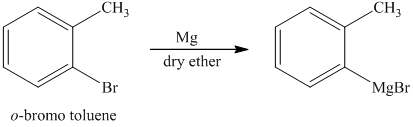
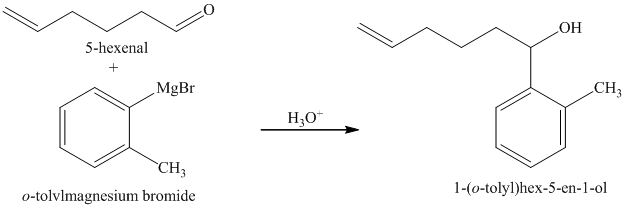
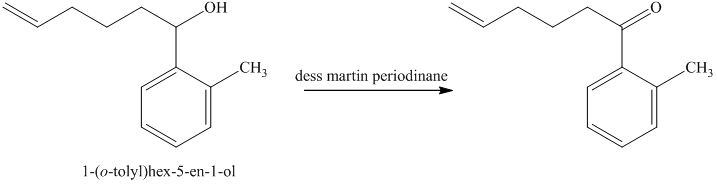
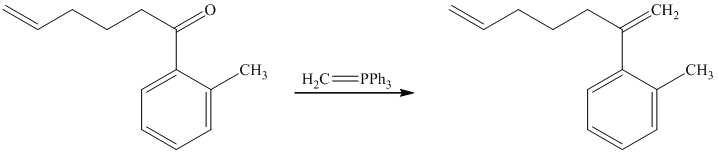
d) Short sequences of reactions for the transformation of the given compounds are shown below.

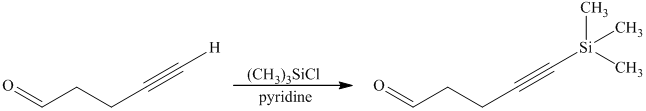
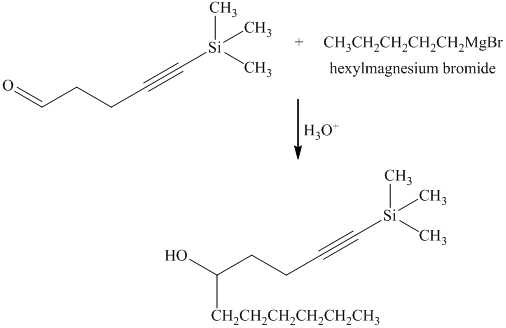
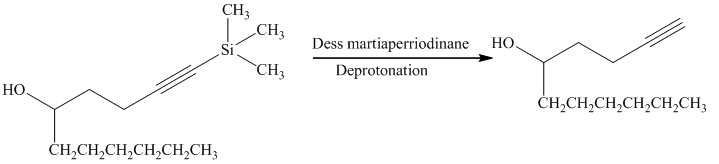
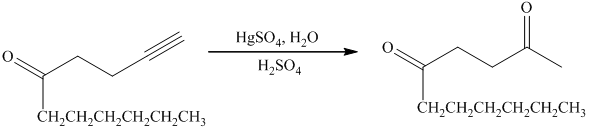
e) Short sequences of reactions that are appropriate for the transformation of the given compound from
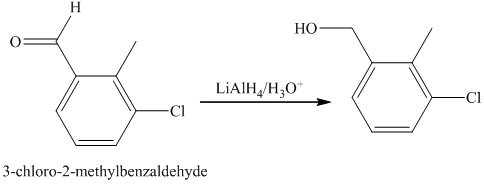
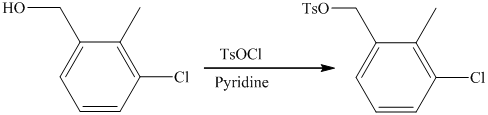
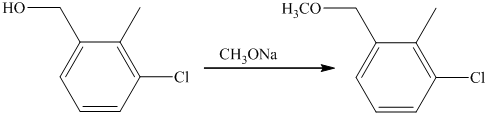
f) Short sequences of reactions that are appropriate for the transformation of the given compounds are shown below.
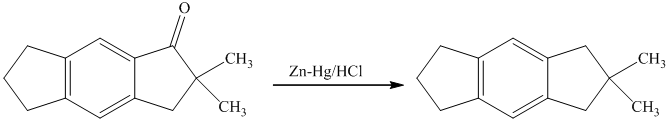
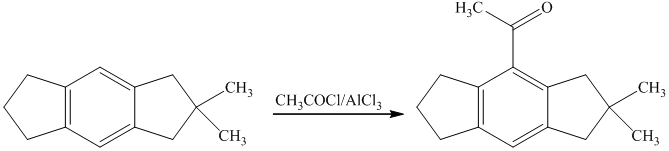
g) Short sequences of reactions that are appropriate for the transformation of the given compounds are shown below.
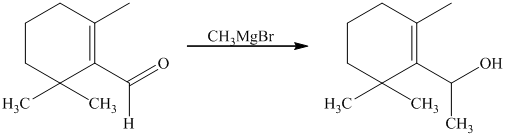
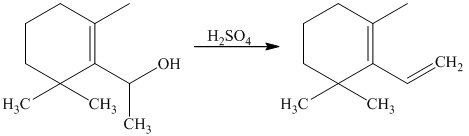
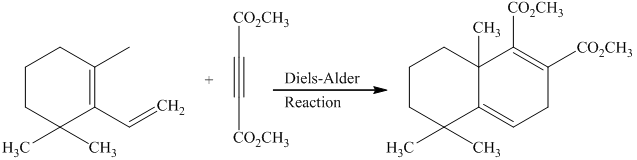
Explanation of Solution
a) Short sequences of reactions that would be appropriate for the transformation of
Treatment of
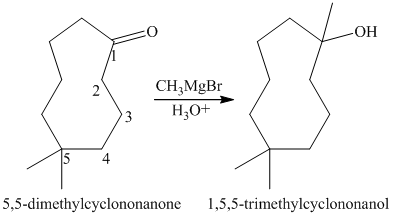
The tertiary alcohol so obtained undergoes dehydration with conc.

Reduction of the alkene with

b) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be decribed.
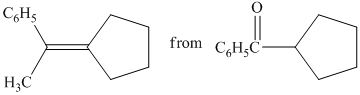
Treatment of cyclopentyl(phenyl) methanone with griginard reagent

The tertiary alcohol, upon dehydration with conc.

c) The structure of the compound which has to be synthesized from
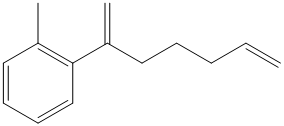
The reaction of

The griginard reagent formed reacts with

The alcohol formed reacts with dess martin periodinane to form ketone as shown in the reaction below.

The ketone reacts with Wittig reagent

d) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be described.
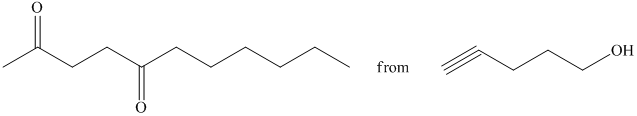
Oxidation of the given alcohol using pyridinium dichromate (PDC) in

Protection of acidic proton of is done by reaction of the aldehyde with

Reaction of the aldehyde with the griginard reagent, hexylmagnesium chloride, produces an alcohol as shown in the reaction below.

Alcohols react with martin dess periodinane to form ketone as shown below.

Terminal alkynes are converted to ketones by reaction with

e) The structure of the compound which has to be synthesized from
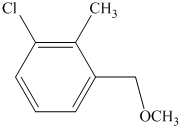
In the reduction of

The hydroxyl group of the alcohol is a poor leaving group and therefore, it is reacted with

The

f) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be decribed.
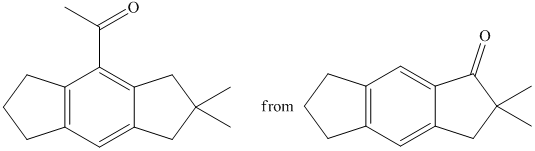
Reduction of the ketone shown above with zinc-amalgam produces a hydrocarbon in Clemmenson’s reduction as shown in the reaction below.

Friedal Crafts acylation of the aromatic ring obtained with

g) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be decribed.
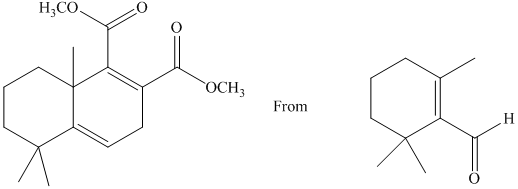
Treatment of the reactant with griginard reagent

The tertiary alcohol so obtained undergoes dehydration with conc.

Diels-Alder reaction of the diene with the dienophile produces the desired product as shown in the reaction below.

Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- Done 18:25 www-awu.aleks.com .III LTE Chapter 12 HW Question 29 of 39 (6 points) | Question Attempt: 1 of Unlimi... Oli 23 24 25 26 27 28 29 30 Consider this structure. CH2 CH2CH2 CH2CH2CH₂ C -C. -CH2CH3 H CH Part: 0 / 3 Part 1 of 3 Give the IUPAC name of this structure. Skip Part < Check ☑ Save For Later © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility ....................arrow_forwardCalculate Ecell at 25.0 oC using the following line notation. Zn(s)|Zn+2(aq, 0.900 M)||Cu+2(aq, 0.000200 M)|Cu(s)arrow_forwardPredict the product of this organic reaction: O OH + H + OH A P + H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click and drag to start drawing a structure. X G ☐ :arrow_forward
- 0.0994 g of oxalic acid dihydrate is titrated with 10.2 mL of potassium permanganate. Calculate the potassium permanganate concentration. Group of answer choices 0.0433 M 0.135 M 0.0309 M 0.193 Marrow_forwardExperts...can any one help me solve these problems?arrow_forwardAccording to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forward
- Which Group 1 metal reacts with O2(g) to form a metal peroxide (M2O2)? Group of answer choices Li K Rb Naarrow_forwardWhich of the following statements is true regarding the reaction between Group 1 metals and water? Group of answer choices These reactions result in a basic solution. The metals do not actually react easily with water due to the metals' lack of conductivity. These reaction result in an acidic solution. The metals need their outer coatings of metal oxides to react.arrow_forwardWhich element cannot interact with hydrogen through hydrogen bonds? Group of answer choices O S Br Narrow_forward
- Which of the following statements is false regarding hydrogen gas production? Group of answer choices Steam reforming requires a catalyst. Methanol (CH3OH) can react with water using a ZnO catalyst to form H2(g). Methanol (CH3OH) can react with O2(g) using a Pd catalyst to form H2(g). The reaction between CH4(g) and H2O to form H2(g) requires a temperature of at least 700 oCarrow_forwardWhich of the following forms of hydrogen is the least stable? Group of answer choices H H2 H− H+arrow_forwardConsider the following reduction half reactions and standard reduction potentials: Fe3+ + e− → Fe2+ Eo = +0.77 V Fe2+ + e− → Fe(s) Eo = -0.44 V Which of the following statements is true? Group of answer choices The Fe2+ reduction to Fe(s) is spontaneous. Fe2+ can disproportionate into Fe3+ and Fe(s) The Fe3+ reduction to Fe2+ is not spontaneous. Fe3+ and Fe(s) can undergo a comproportionation reaction to form Fe2+arrow_forward

 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


