
Draw the structure for each of the following:
a. phenol
b. benzyl phenyl ether
c. benzonitrile
d. benzaldehyde
e. anisole
f. styrene
g. toluene
h. tert-buty lbenzene
i. benzyl chloride
a)
Interpretation:
The structure of phenol is to be drawn.
Concept introduction:
Phenols are defined as those compounds in which hydroxy group is attached directly to the benzene ring. Phenols and alcohols have so many similar properties.
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of phenol is shown below.
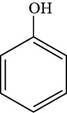
Figure 1
Explanation of Solution
The structure of phenol is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of phenol is shown below.
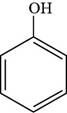
Figure 1
b)
Interpretation:
The structure of benzyl phenyl ether is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzyl phenyl ether is shown below.
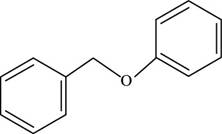
Figure 2
Explanation of Solution
The structure of benzyl phenyl ether is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzyl phenyl ether is shown below.

Figure 2
c)
Interpretation:
The structure of benzonitrile is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzonitrile is shown below.
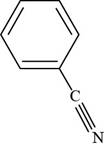
Figure 3
Explanation of Solution
The structure of benzonitrile is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzonitrile is shown below.
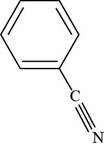
Figure 3
d)
Interpretation:
The structure of benzaldehyde is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzaldehyde is shown below.
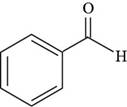
Figure 4
Explanation of Solution
The structure of benzaldehyde is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzaldehyde is shown below.
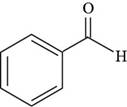
Figure 4
e)
Interpretation:
The structure of anisole is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of anisole is shown below.
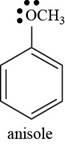
Figure 5
Explanation of Solution
The structure of anisole is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of anisole is shown below.
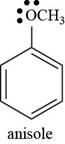
Figure 5
f)
Interpretation:
The structure of styrene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of styrene is shown below.
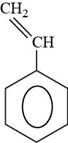
Figure 6
Explanation of Solution
The structure of styrene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of styrene is shown below.
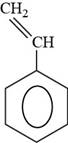
Figure 6
g)
Interpretation: The structure of toluene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of toluene is shown below.
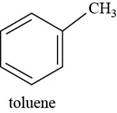
Figure 7
Explanation of Solution
The structure of toluene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of toluene is shown below.
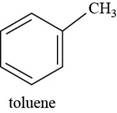
Figure 7
h)
Interpretation: The structure of tert-butyl benzene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of tert-butyl benzene is shown below.
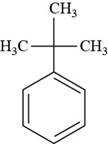
Figure 8
Explanation of Solution
The structure of tert-butyl benzene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of tert-butyl benzene is shown below.

Figure 8
i)
Interpretation:
The structure of benzyl chloride is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzyl chloride is shown below.
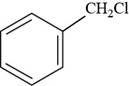
Figure 9
Explanation of Solution
The structure of benzyl chloride is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzyl chloride is shown below.

Figure 9
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry (8th Edition)
- Predict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Erase something Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terarrow_forwardQ14. Fill this chart: (please refer to ppt notes/browser to answer these questions) What alcohol is also called wood alcohol? What is the common name of ethanol? Draw the structure of phenol and thiophene? Are bigger chain alcohol like heptanol and octanol are soluble or insoluble in water and explain it ? Are ethers soluble or insoluble in water? What suffix and prefix are used for alcohol while naming alcohol and ether? What the process called when we add water to any alkene to make alcohol? Q16. Draw the diagram of following aromatic compound (practice from previous module) Aniline Phenol Benzoic acid Methyl benzoate Q17. a. Write the oxidation reactions for the 2 propanol. b. Write the oxidation reaction of the ethanol.arrow_forwardQuestion 11 of 18 (1 point) Question Attempt: 3 of How many signals do you expect in the 'H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: In this question, any multiplet is counted as one signal. Number of signals in the 'H NMR spectrum. 1 For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in top molecule Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box…arrow_forward
- Organic Chemistry Esterification reactions 1. Write the steps to prepare ester. 2. Write complete reaction of ethanol and acetic acid to make ester. 3. What does ester smell like? What are the uses of ester. 4. What the role of sulfuric acid in the esterification reactionarrow_forward11. Complete the following esterification reaction with names of all the reactants and products under. Hint: Remove the water and end up with ester R-C-OH + ROH R-C-OR + H₂O A carboxylic acid An alcohol An ester Water BYJU'S H-C-C O-H Нин C-C-C-H HAAA H O-C-C-C-H AAA Ethanoic acid Propanol Water Propyl ethanoate By com CH3COOH + CH3CH2CH2CH₂CH₂OH → Practice for alcohols aldehydes and ketones: 12. Draw the structures from the following names mixed of alcohol/aldehyde and ketone: a. 4-methyl cyclohexanone b. 3-methyl-2-pentenal c. 2,3-dimethylcyclohexanone d. 1,3propanediol or Propane 1,3 diol 13. Write systematic names for the following compounds identify functional group: a. b. (CH3)2CH-C OH c) CH(CH₂)-- OH -,-,arrow_forwardmay you please show all steps! i am having a hard time understanding and applying in this format, thank you!arrow_forward
- 10. Complete the substitution reaction of 2 pentanol with these reagents. Reagents & Reaction Conditions use practice sheet. Please write only major products, minor product like water, other gases are not required. Hint: In substitution of alcohol, we generally substitute OH group with Halogens like cl, Br, F using some reagent containing halogens. Ensure to add halogens to the same carbon number where you are removing OH from Examples Alcohols can be converted to Alkyl Halides with HX acids HBr H₂O HCI + H₂O HI + H₂O CH,CH₂OH + SOCI₂ CH,CH₂OH + PCI₁₂ A BBYJU'S CH CHCI + SO₂+ HCI CH₂CH CIP(OH), + HCI CH,CH₂OH + PCI CHCHCI + POCI + HCI CH,CH₂OH + PBr, CH,CH,Br + P(OH), + HBr 1. Reaction with HBr with 2 Pentanol 2.Reaction with HI with 2 pentanol © Byjus.com 3.Reaction with HCI+ZnCl,, with 2 pentanol (Zncl2 is catalyst no role) 4.Reaction with SOCI,, with 2 Pentanol 5.Reaction with PBr; or PCl, with 2 pentanolarrow_forward3. Is 2-methyl-2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-methyl-2-propanol and also any oxidation products of 2- methyl-2- propanol. If there is more than one oxidation product, give the structure of each of the products. 4. 2-Propanol is the IUPAC systematic name of this alcohol. It has a common name by which it is much better known (You'll see it in the grocery store or pharmacy). Give that common name 5. Aldehydes can be synthesized by the oxidation of. Please choose from below choices A. Primary alcohols B. Secondary alcohols C. Organic acids D. Inorganic acids 6. Tertiary alcohol Can undergo oxidation. yes or no. ? If yes then answer the product.arrow_forwardFinish the reactions hand written pleasearrow_forward
- Part A Identify each alcohol as primary, secondary, or tertiary Drag the appropriate items to their respective bins. CH₂ H₂C- -C-OH HO CH₂ Primary Он OH CH₂ OH CCH₂OH CH₂ сн Secondary Tertiary Reset Help CH,CH₂ (CH)CHCH,OH CH,CH,CH,CCH, CHOH CH₂ Different types of alcohol groups Alcohol and its reaction: 8. Combing two alcohol molecules below and completing the reaction with Product .( Hint Reaction called etherification as ether is formed and name the ether once you complete the reaction. Hint.: R-O-H+H-O-RR-O-R Do the reaction: CH₂OH + CH₂OH---→ + H-O-H 9. Write the reaction of formation of alcohol from alkene by adding water: Addition reaction also called hydration reaction as we are adding water which occur always in presence of acid Hint: Break the double bond and add H and OH if symmetrical then add anywhere if unsymmetrical then follow Markovnikov rule H should go to that double bone carbon which has more hydrogen CH2=CH2 + H₂O-→arrow_forwardComplete the reaction hand written pleasearrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. dm Re Explanation Check ©2025 McGraw Hill LLC. All Rights Reserved. Termarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
