
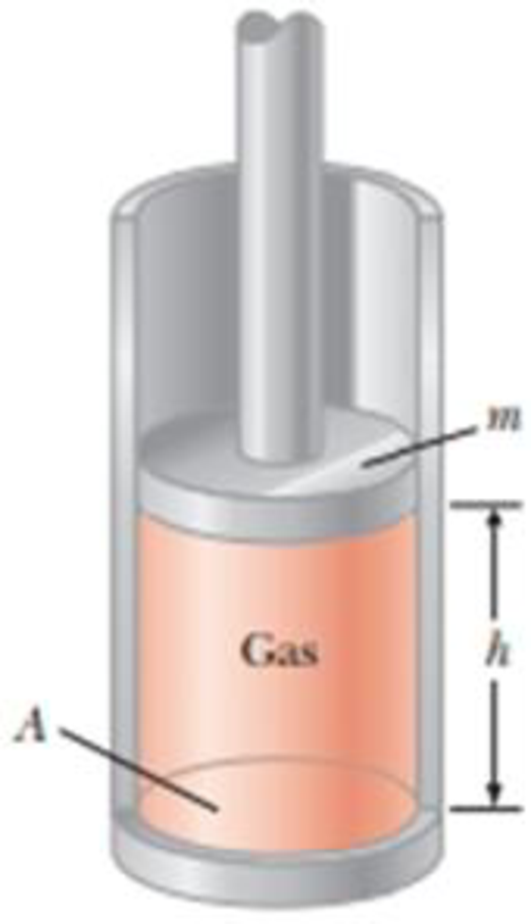
A vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless piston of mass m (Fig. P18.40). The piston is not restricted in its motion in any way and is supported by the gas at pressure P below it. Atmospheric pressure is P0. We wish to find the height h in Figure P18.40. (a) What analysis model is appropriate to describe the piston? (b) Write an appropriate force equation for the piston from this analysis model in terms of P, P0, m, A, and g. (c) Suppose n moles of an ideal gas are in the cylinder at a temperature of T. Substitute for P in your answer to part (b) to find the height h of the piston above the bottom of the cylinder.
Figure P18.40
Trending nowThis is a popular solution!

Chapter 18 Solutions
Physics for Scientists and Engineers with Modern Physics
- Part 2 - Geometry and Trigonometry 1. Line B touches the circle at a single point. Line A extends radially through the center of the circle. A B (a) Which line is tangential to the circumference of the circle? (b) What is the angle between lines A and B. 2. In the figure below what is the angle C? 30 45 3. In the figure below what is the value of the angle 0? 30° 4. In the figure below what is the value of the angle 0? A 30°arrow_forwardDetails solution No chatgpt plsarrow_forwardPlease solve and answer the problem correctly please.Thank you!!arrow_forward
- Will you please walk me through the calculations in more detail for solving this problem? I am a bit rusty on calculus and confused about the specific steps of the derivation: https://www.bartleby.com/solution-answer/chapter-3-problem-15e-modern-physics-2nd-edition/9780805303087/7cf8c31d-9476-46d5-a5a9-b897b16fe6fcarrow_forwardplease help with the abstract. Abstract - This document outlines the format of the lab report and describes the Excel assignment. The abstract should be a short paragraph that very briefly includes the experiment objective, method, result and conclusion. After skimming the abstract, the reader should be able to decide whether they want to keep reading your work. Both the format of the report and the error analysis are to be followed. Note that abstract is not just the introduction and conclusion combined, but rather the whole experiment in short including the results. I have attacted the theory.arrow_forwardUsing the Experimental Acceleration due to Gravity values from each data table, Data Tables 1, 2, and 3; determine the Standard Deviation, σ, mean, μ, variance, σ2 and the 95% Margin of Error (Confidence Level) Data: Ex. Acc. 1: 12.29 m/s^2. Ex. Acc. 2: 10.86 m/s^2, Ex. Acc. 3: 9.05 m/s^2arrow_forward
- In the Super Smash Bros. games the character Yoshi’s has a “ground pound” down special move where he launches himself downward to attack an enemy beneath him. A) If Yoshi flings himself downwards at 9.76 miles per hour to hit an enemy 10.5 m below him, how fast is Yoshi traveling when he hits the enemy? 1 mile = 1609 m B) How much time does it take Yoshi to hit the enemy beneath him?arrow_forwardNo chatgpt pls will upvotearrow_forwardSolve No chatgpt pls will upvotearrow_forward
- Can someone help me solve this thank you.arrow_forwardNo chatgpt pls will upvotearrow_forward1.62 On a training flight, a Figure P1.62 student pilot flies from Lincoln, Nebraska, to Clarinda, Iowa, next to St. Joseph, Missouri, and then to Manhattan, Kansas (Fig. P1.62). The directions are shown relative to north: 0° is north, 90° is east, 180° is south, and 270° is west. Use the method of components to find (a) the distance she has to fly from Manhattan to get back to Lincoln, and (b) the direction (relative to north) she must fly to get there. Illustrate your solutions with a vector diagram. IOWA 147 km Lincoln 85° Clarinda 106 km 167° St. Joseph NEBRASKA Manhattan 166 km 235° S KANSAS MISSOURIarrow_forward
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





