
BURDGE CHEMISTRY VALUE ED (LL)
4th Edition
ISBN: 9781259995958
Author: VALUE EDITION
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 3KSP
The diagram shown here depicts a system at equilibrium for the reaction
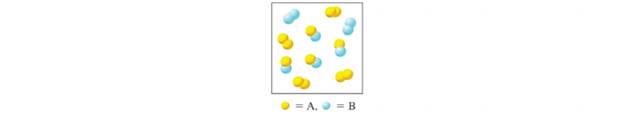
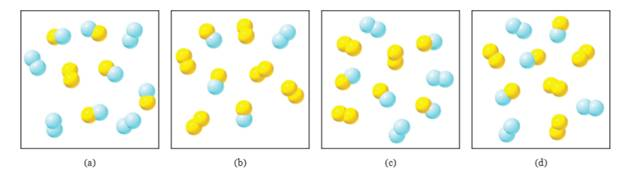
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 18 Solutions
BURDGE CHEMISTRY VALUE ED (LL)
Ch. 18.1 - Practice Problem ATTEMPT
Determine the change in...Ch. 18.1 - Practice Problem BUILD To what fraction of its...Ch. 18.1 - Practice Problem CONCEPTUALIZE
Which equation is...Ch. 18.2 - Practice ProblemATTEMPT Calculate the standard...Ch. 18.2 - Practice Problem BUILD
In each of the following...Ch. 18.2 - Practice Problem CONCEPTUALIZE
For each reaction...Ch. 18.3 - Practice ProblemATTEMPT For each of the following...Ch. 18.3 - Practice Problem BUILD
Make a qualitative...Ch. 18.3 - Practice Problem CONCEPTUALIZE
Consider the...Ch. 18.3 - 18.3.1 For which of the following physical...
Ch. 18.3 - 18.3.2 For which of the following chemical...Ch. 18.3 - 18.3.3 Identify the correct balanced equation and...Ch. 18.4 - Practice Problem ATTEMPT For each of the...Ch. 18.4 - Practice Problem BUILD (a) Calculate Δ S univ and...Ch. 18.4 - Practice Problem CONCEPTUALIZE The following table...Ch. 18.4 - Using data from Appendix 2, calculate Δ S ° (in...Ch. 18.4 - 18.4.2 Using data from Appendix 2, calculate (in...Ch. 18.4 - The diagrams show a spontaneous chemical reaction....Ch. 18.4 - 18.4.4 The diagrams show a spontaneous chemical...Ch. 18.5 - Practice Problem ATTEMPT
A reaction will be...Ch. 18.5 - Practice Problem BUILD
Given that the reaction is...Ch. 18.5 - Practice ProblemCONCEPTUALIZE Which of the...Ch. 18.5 - A reaction for which Δ H and Δ S are both negative...Ch. 18.5 - At what temperature ( in ºC ) does a reaction go...Ch. 18.5 - 18.5.3 Using data from Appendix 2, calculate G°...Ch. 18.5 - 18.5.4 Calculate for the sublimation of iodine in...Ch. 18.6 - Practice Problem ATTEMPT
Calculate the standard...Ch. 18.6 - Practice problemBUILD For each reaction, determine...Ch. 18.6 - Prob. 1PPCCh. 18.6 - 18.6.1 For the reaction:
Ch. 18.6 - Consider the reaction: X ( g ) + Y(g) ⇄ Z( g ) for...Ch. 18.6 - The Δ G° for the reaction: N 2 ( g ) + 3H 2 (g) ⇄...Ch. 18.6 - 18.6.4 The for iron(III) hydroxide . For the...Ch. 18.7 - Practice Problem ATTEMPT
The molar heats of fusion...Ch. 18.7 - Practice Problem CONCEPTUALIZE
Explain why. in...Ch. 18.8 - Practice ProblemATTEMPT Δ G ° for the reaction: H...Ch. 18.8 - Practice ProblemBUILD What is the minimum partial...Ch. 18.8 - Practice Problem CONCEPTUALIZE Consider the...Ch. 18.9 - Practice Problem ATTEMPT Using data from Appendix...Ch. 18.9 - Practice ProblemBUILD K f for the complex ion Ag (...Ch. 18.9 - Practice Problem CONCEPTUALIZE Which of the...Ch. 18.10 - Practice ProblemATTEMPT Calculate G for the...Ch. 18.10 - Practice ProblemBUILD Ksp for Co(OH)2 at...Ch. 18.10 - Prob. 1PPCCh. 18 - 18.1
Which of the following must be negative for a...Ch. 18 - Δ G for a reaction is always negative when (a) Δ G...Ch. 18 - 18.3
The diagram shown here depicts a system at...Ch. 18 - The reaction shown here has Δ G º = -1 .83 kJ/mol...Ch. 18 - 18.1 Explain what is meant by a spontaneous...Ch. 18 - Prob. 2QPCh. 18 - Prob. 3QPCh. 18 - Describe what is meant by the term entropy. What...Ch. 18 - Prob. 5QPCh. 18 - Prob. 6QPCh. 18 - Prob. 7QPCh. 18 - Prob. 8QPCh. 18 - How does the entropy of a system change for each...Ch. 18 - Prob. 10QPCh. 18 - Prob. 11QPCh. 18 - Prob. 12QPCh. 18 - Prob. 13QPCh. 18 - Using the data in Appendix 2, calculate the...Ch. 18 - 18.15 Using the data in Appendix 2, calculate the...Ch. 18 - Prob. 16QPCh. 18 - Prob. 17QPCh. 18 - Prob. 18QPCh. 18 - 18.19 State the third law of thermodynamics in...Ch. 18 - Calculate Δ S surr for each of the reactions in...Ch. 18 - Calculate Δ S surr for each of the reactions in...Ch. 18 - Using data from Appendix 2, calculate Δ S rxn º...Ch. 18 - 18.23 Using data from Appendix 2, calculate for...Ch. 18 - Prob. 24QPCh. 18 - Why is it more convenient to predict the direction...Ch. 18 - What is the significance of the sign of Δ G sys ?Ch. 18 - From the following combinations of Δ H and Δ S ,...Ch. 18 - Prob. 28QPCh. 18 - Prob. 29QPCh. 18 - From the values of Δ H and Δ S , predict which of...Ch. 18 - Find the temperatures at which reactions with the...Ch. 18 - The molar heats of fusion and vaporization of...Ch. 18 - 18.33 The molar heats of fusion and vaporization...Ch. 18 - Prob. 34QPCh. 18 - Prob. 35QPCh. 18 - Prob. 36QPCh. 18 - Prob. 37QPCh. 18 - Prob. 38QPCh. 18 - Explain why Equation 18.14 is of great importance...Ch. 18 - Prob. 40QPCh. 18 - Prob. 41QPCh. 18 - Prob. 42QPCh. 18 - 18.43 Consider the following reaction at...Ch. 18 - Prob. 44QPCh. 18 - 18.45
(a)
Calculate and for the following...Ch. 18 - Prob. 46QPCh. 18 - Consider the decomposition of calcium carbonate:...Ch. 18 - Prob. 48QPCh. 18 - 18.49 At for the process:
is 8.6 kJ/mol....Ch. 18 - Prob. 50QPCh. 18 - What is a coupled reaction? What is its importance...Ch. 18 - What is the role of ATP in biological reactions?Ch. 18 - Prob. 53QPCh. 18 - 18.54 In the metabolism of glucose, the first step...Ch. 18 - Predict the signs of Δ H , Δ S , and Δ G of the...Ch. 18 - Prob. 56APCh. 18 - Prob. 57APCh. 18 - Prob. 58APCh. 18 - Prob. 59APCh. 18 - Prob. 60APCh. 18 - Ammonium nitrate ( NH 4 NO 3 ) dissolves...Ch. 18 - 18.62 Calculate the equilibrium pressure of due...Ch. 18 - Prob. 63APCh. 18 - Referring to Problem 18.63, explain why the ratio...Ch. 18 - 18.65 Which of the following are not state...Ch. 18 - 18.66 For reactions carried out under...Ch. 18 - Prob. 67APCh. 18 - Prob. 68APCh. 18 - A student looked up the Δ G f o , Δ H f o , and Δ...Ch. 18 - Consider the following Brønsted acid-base reaction...Ch. 18 - 18.71 At o K, the entropy of carbon monoxide...Ch. 18 - Prob. 72APCh. 18 - Consider the thermal decomposition of CaCO 3 :...Ch. 18 - Prob. 74QPCh. 18 - Prob. 75QPCh. 18 - Prob. 76QPCh. 18 - Prob. 77APCh. 18 - Prob. 78APCh. 18 - Prob. 79APCh. 18 - Prob. 80APCh. 18 - Prob. 81APCh. 18 - Prob. 82APCh. 18 - 18.83 Comment on the statement: “Just talking...Ch. 18 - Prob. 84APCh. 18 - Consider the reaction: N 2 ( g ) + O 2 ( g ) ⇄ 2...Ch. 18 - Prob. 86APCh. 18 - Consider the decomposition of magnesium carbonate:...Ch. 18 - Prob. 88APCh. 18 - Prob. 89APCh. 18 - 18.90 The rate constant for the elementary...Ch. 18 - A 74.6-g ice cube floats in the Arctic Sea. The...Ch. 18 - 18.92 Which of the following is not accompanied by...Ch. 18 - Prob. 93APCh. 18 - Give a detailed example of each of the following,...Ch. 18 - Prob. 95QPCh. 18 - 18.96 The standard enthalpy of formation and the...Ch. 18 - Prob. 97QPCh. 18 - Prob. 98QPCh. 18 - The following reaction was described as the cause...Ch. 18 - Comment on the feasibility of extracting copper...Ch. 18 - 18.101 One of the steps in the extraction of iron...Ch. 18 - Prob. 102APCh. 18 - Prob. 103APCh. 18 - Prob. 104APCh. 18 - 18.105 The enthalpy change in the denaturation of...Ch. 18 - Prob. 106APCh. 18 - Prob. 107APCh. 18 - Prob. 108APCh. 18 - Prob. 109APCh. 18 - Prob. 110APCh. 18 - 18.111 Carbon monoxide and nitric oxide are...Ch. 18 - Prob. 112APCh. 18 - Prob. 113APCh. 18 - 18.114 Many hydrocarbons exist as structural...Ch. 18 - Physical and Biological Sciences
In chemistry, the...Ch. 18 - Physical and Biological Sciences
In chemistry, the...Ch. 18 - Prob. 3SEPPCh. 18 - Physical and Biological Sciences
In chemistry, the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY