
EBK ESSENTIAL ORGANIC CHEMISTRY
3rd Edition
ISBN: 9780100659469
Author: Bruice
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 36P
For each of the following reaction, name both the enzyme that catalyzes the reaction and the required coenzyme:
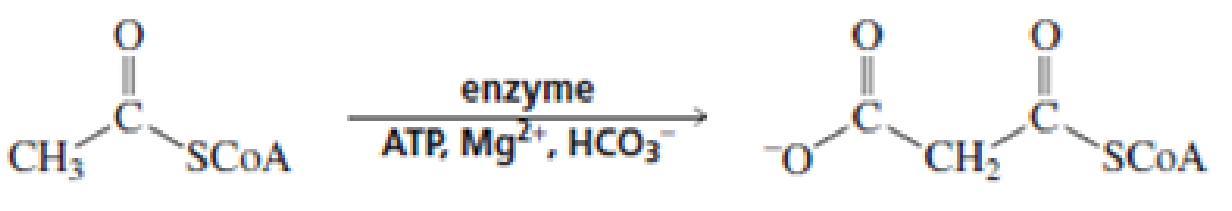

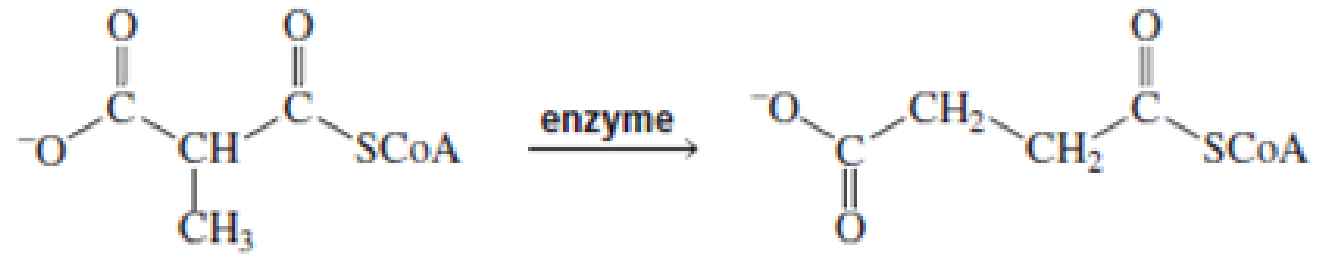
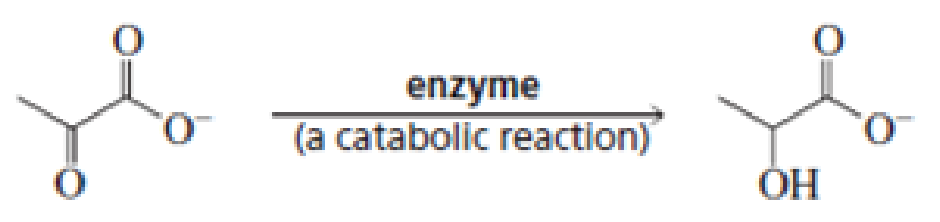
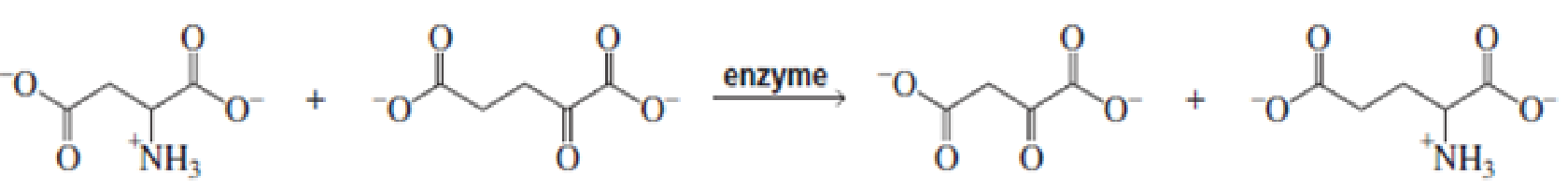

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Chapter 18 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
Ch. 18.1 - Prob. 1PCh. 18.2 - If H218O were used to hydrolyze lysozyme, which...Ch. 18.3 - Which of the following amino acid side chains can...Ch. 18.3 - Arginine and lysine side chains fit into trypsins...Ch. 18.4 - Which of the following amino acid side chains can...Ch. 18.4 - Prob. 6PCh. 18.5 - Prob. 7PCh. 18.5 - Draw the mechanism for the hydroxide-ion-catalyzed...Ch. 18.5 - What advantage does the enzyme gain by forming an...Ch. 18.7 - Prob. 10P
Ch. 18.7 - Prob. 11PCh. 18.8 - How many conjugated double bonds are there in a....Ch. 18.8 - Instead of adding to the 4a-position and...Ch. 18.8 - In succinate dehydrogenase, FAD is covalently...Ch. 18.8 - Prob. 15PCh. 18.9 - Acetolactate synthase is another TPP-requiring...Ch. 18.9 - Acetolactate synthase can also transfer the acyl...Ch. 18.9 - Prob. 18PCh. 18.9 - Prob. 19PCh. 18.10 - Prob. 21PCh. 18.11 - Prob. 23PCh. 18.11 - Which compound is more easily decarboxylated?Ch. 18.11 - Explain why the ability of PLP to catalyze an...Ch. 18.11 - Explain why the ability of PLP to catalyze an...Ch. 18.12 - What groups are interchanged in the following...Ch. 18.13 - Why is the coenzyme called tetrahydrofolate?Ch. 18.13 - What amino acid is formed by the following...Ch. 18.13 - How do the structures of tetrahydrofolate and...Ch. 18.13 - What is the source of the methyl group in...Ch. 18 - Prob. 32PCh. 18 - Prob. 33PCh. 18 - From what vitamins are the following coenzymes...Ch. 18 - Prob. 35PCh. 18 - For each of the following reaction, name both the...Ch. 18 - Explain why serine proteases do not catalyze...Ch. 18 - Prob. 38PCh. 18 - For each of the following enzyme catalyzed...Ch. 18 - Trisephosphate isomerase (TIM) catalyzes the...Ch. 18 - Prob. 41PCh. 18 - What acyl groups have we seen transferred by...Ch. 18 - When UMP is dissolved in T2O, exchange of T for H...Ch. 18 - Prob. 44PCh. 18 - When transaminated, the three branched-chain amino...Ch. 18 - Aldolase shows no activity if it is incubated with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forward
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY