
Concept explainers
Interpretation: The structures of cyclohexa-2, 4-dien-1-one and its enol form are to be written, and the special factors that account for the stability of the enol form are to be identified.
Concept introduction:
Tautomerization is defined as the interconversion of the keto and enol forms. The keto and enol forms are tautomers.
The conjugate base of both the keto and the enol forms is enolate.
If the enolate accepts a proton from carbon, the keto form is formed, and if the enolate accepts a proton from oxygen, the enol form is formed.
Answer to Problem 1PP
Solution:
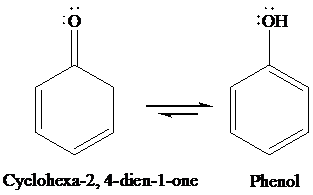
Due to aromaticity.
Explanation of Solution
Under acidic condition, cyclohexa-2, 4-dien-1-one converts to phenol through tautomerism. The structures of cyclohexa-2, 4-dien-1-one and its enol form are as follows:
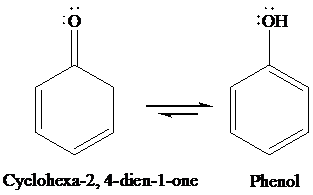
Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


