
Concept explainers
(a)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of
Answer to Problem 1P
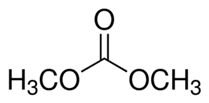
Explanation of Solution
We know that
(b)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 1P
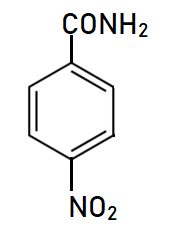
Explanation of Solution
This is a nitro derivative of benzamide in which nitro group is bonded at para position with respect to amide group. Benzamide is
(c)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 1P
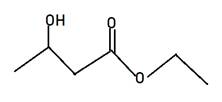
Explanation of Solution
Alkyl alkanoate is the general name for ester. Here Alkyl indicates the small alkyl group in RCOOR and alkanoate is RCOO- part. The name given is ethyl 3-hydroxybutanate, hence 3-hydroxybutanate will be RCOO-part and ethyl will be another r of ester. Therefore, the structure of ethyl 3-hydroxybutanate will be
(d)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 1P
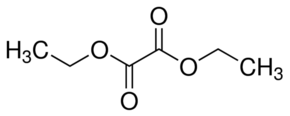
Explanation of Solution
Diethyl oxalate is the diester of oxalic acid (HOOC-COOH). As name suggested, diethyl stands for two ethyl group bonded at both carbon atoms of oxalic acid hence the formula will be
(e)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 1P
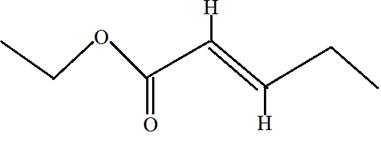
Ethyl trans-2-pentenoate.
Explanation of Solution
Ethyl trans-2-pentenoate is the ester of 2-pentenoic acid with ethanol. Here trans indicates the position of H on both double bonded carbon atoms. Hence in the formula of ester R1 -COO-R2 ; R2 will be ethyl group from alcohol and R1 will be alkyl group from acid.
(f)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 1P
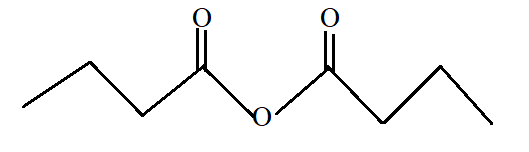
Butanoic anhydride.
Explanation of Solution
Butanoic anhydride is the anhydride of butanoic acid with −COOCO- as
Want to see more full solutions like this?
Chapter 18 Solutions
EP INTRO.TO GENERAL,ORGANIC...-OWL ACCE
- Select the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forwardb. H3C CH3 H3O+ ✓ H OHarrow_forward
- 2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forward
- Instructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forwardC. NaOMe, Br Brarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
