
Concept explainers
(a)
Interpretation:
The amine needs to be labeled as 10, 20 or 30.
Concept introduction:
Answer to Problem 18.81P
A tertiary (3o) amine.
Explanation of Solution
Amines are derivatives which are derived from ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. They can be called as alkylamines and arylamines.
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced, it is known as a tertiary amine.
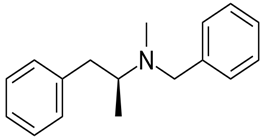
Here, all three hydrogen atoms are replaced and therefore this amine called a tertiary (3o) amine.
(b)
Interpretation:
The chiral center of the benzphetamine molecule needs to be labeled.
Concept Introduction:
The molecules with a chiral center can form a superimposable mirror image and is known as enantiomers. There should not be any plane of symmetry in a molecule to be chiral. A plane that bisects a molecule into two equal halves is known as a plane of symmetry. If there is a plane of symmetry in a molecule and it is identical to any of its mirror image, it is considered as achiral. The chiral center is carbon attached to 4 different groups attached to it.
Answer to Problem 18.81P
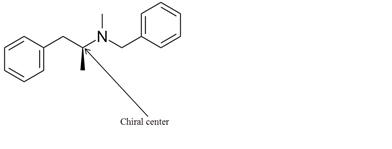
Explanation of Solution
The chiral center in the given molecule is shown as follows:
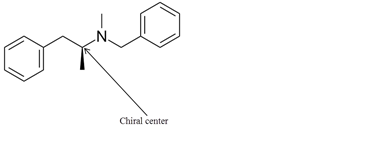
The labeled carbon atom is attached to 4 different groups.
The chiral center has four different groups attached to carbon and no plane of symmetry. The molecule with a chiral center shows optical isomerism. The chiral center is a stereocenter holding the atoms in space, the mirror image so formed are not superimposable.
(c)
Interpretation:
The enantiomers of benzphetamine molecule needs to be determined.
Concept introduction:
The chiral molecules which are mirror images of each other are known as enantiomers. They are non-superimposable mirror images, or they cannot be placed on top of each other.
Answer to Problem 18.81P
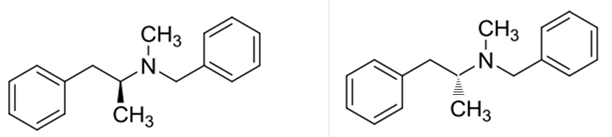
Explanation of Solution
Benzphetamine has two enantiomers due to its one chiral center. It rotates the plane-polarized light. Here, S-benzphetamine rotates the light anticlockwise and R- benzphetamine rotates it clockwise.
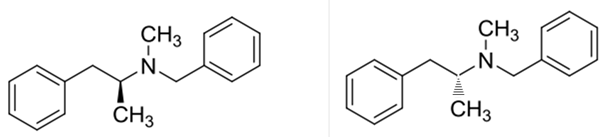
Benzphetaminecan can be called as a racemic mixture of two enantiomers, which are R(+) and S(-).
(d)
Interpretation:
The constitutional isomer that contains a primary amine needs to be drawn.
Concept introduction:
Constitutional isomers have the same molecular formula but different structural connectivity. The number of each atom in both the molecules needs to be counted and the arrangement is observed to check whether two molecules are a constitutional isomer of each other or not.
Answer to Problem 18.81P
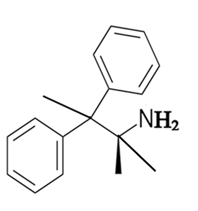
Explanation of Solution
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.
The given compound is as follows:
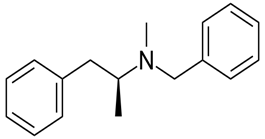
The constitutional isomer with a primary amine is as follows:
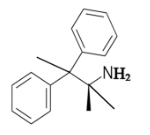
Here, only one hydrogen atom is replaced therefore this amine called a primary amine (1o).
(e)
Interpretation:
The constitutional isomer that contains a tertiary amine needs to be determined.
Concept introduction:
Constitutional isomers have the same molecular formula but different structural connectivity. The number of each atom in both the molecules needs to be counted and the arrangement is observed to check whether two molecules are a constitutional isomer of each other or not.
Answer to Problem 18.81P

Explanation of Solution
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.
The given compound is as follows:
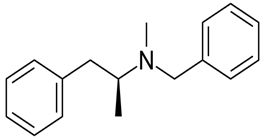
The constitutional isomer with a tertiary amine is represented as follows:
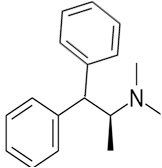
Here all 3 hydrogen atomsare replaced and therefore this amine called as a tertiary amine (3o).
(f)
Interpretation:
The structure of benzphetaminehychloride molecule needs to be drawn.
Concept introduction:
Benzphetamine hydrochloride can be defined as the hydrochloride salt version of benzphetamine.
Answer to Problem 18.81P
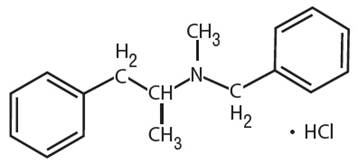
Explanation of Solution
Benzphetamine hydrochloride can be defined as the hydrochloride salt version of benzphetamine. Its molecular formula is C17H22ClN. It has the following structure.
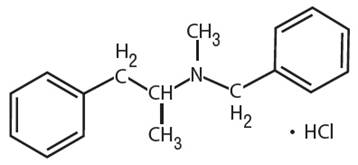
(g)
Interpretation:
The products formed if benzphetamine is treated with acetic acid needs to be determined.
Concept introduction:
Amines are derived from ammonia and formed by replacement of one or more hydrogen atoms by alkyl or aryl groups. They can be called as alkylamines and arylamines. Benzphetamine is a tertiary amine.
Answer to Problem 18.81P
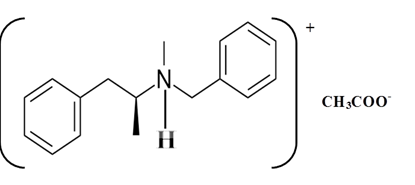
Explanation of Solution
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.
Benzphetamine is a tertiary ammine. It has basic properties and it accepts protons from acids. Acetic acid donates a proton to this amine and formed below the salt.
Want to see more full solutions like this?
Chapter 18 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
- What are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardA block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forwardPotential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forward
- Can u help me figure out the reaction mechanisms for these, idk where to even startarrow_forwardHi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forwardHi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward
- 5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forward
- Draw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole



