
(a)
Interpretation:
The structure for the eight constitutional isomers of molecular formula C4H11N should be drawn.
Concept Introduction:
There are three types of
Answer to Problem 18.48P
The structures for the eight constitutional isomers of molecular formula C4H11N are represented as follows:

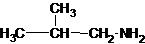
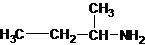
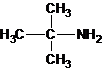
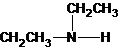
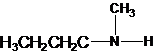
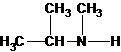
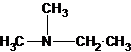
Explanation of Solution
Four structures of primary amines can be drawn with the formula C4H11N.

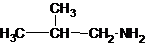
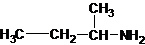
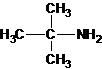
Three structures of secondary amines can also be drawn.
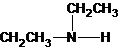
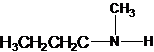
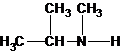
A tertiary structure can also be drawn as follows:
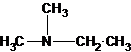
(b)
Interpretation:
The systematic name for each amine should be given.
Concept Introduction:
In nomenclature of primary amine, the longest carbon chain bonded to nitrogen is determined and the −e ending of the parent
Answer to Problem 18.48P
The name of amines are as follows:

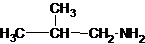
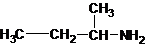
1-butanamine 2-methylpropan-1-amine butan-2-amine
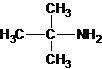
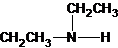
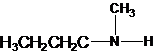
2-methylpropan-2-amine N-ethylethanamine N-methylpropan-1-amine
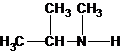
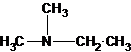
N-methylpropan-2-amine N,N-dimethylethanamine
Explanation of Solution

The longest carbon chain has four carbons. So the alkane name is butane. N is attached to C-1. Therefore, the systematic name of the amine is butanamine.
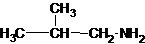
The longest carbon chain has three carbons. There is a methyl group at C-2. So the parent name is 2-methylpropanamine. The N atom is bonded to C-1. Therefore, the name become 2-methylpropan-1-amine.
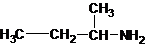
The longest carbon chain bonded to amine group has four carbons. The parent name is butanamine. The N atom is bonded to C-2. Therefore, the systematic name of the amine is butan-2-amine.
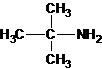
The longest carbon chain bonded to amine group has three carbons. There is a methyl group at C-2. The parent name is 2-methylpropanamine. The N atom is bonded to C-2. Therefore, the systematic name of the amine is 2-methylpropan-2-amine.
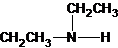
The secondary amine has the longest carbon chain with 2 carbons. So, the parent name is ethanamine. The N atom has bonded to C-1 and has 1 ethyl group as a substituent. Therefore, the systematic name become N-ethylethanamine.
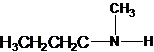
The secondary amine has the longest carbon chain with 3 carbons. So, the parent name is propanamine. The N atom has bonded to C-1 and has 1 methyl group as a substituent. Therefore, the systematic name become N-methylpropan-1-amine.
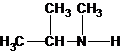
The secondary amine has the longest carbon chain with 3 carbons. So, the parent name is propanamine. The N atom has bonded to C-2 and has 1 methyl group as a substituent. Therefore, the systematic name become N-methylpropan-2-amine.
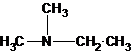
The tertiary amine has the longest carbon chain with 2 carbons. So the parent name is ethanamine. N atom has bonded to C-1 and has two methyl groups and 1 ethyl group as substituents. So, the systematic name of the amine is N,N-dimethylethanamine.
(c)
Interpretation:
The chirality center present in one of the amines should be identified.
Concept Introduction:
An atom that has four different groups bonded to it is referred to as chirality center. A chiral molecule has a non-superimposable mirror image.
Answer to Problem 18.48P
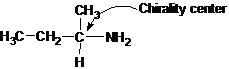
Explanation of Solution
Butan-2-amine has long carbon chain with 4 carbons and amine group is bonded to C-2. This C-2 carbon has four different groups bonded to it as 1 ethyl group, 1 methyl group, 1 amine group and a hydrogen. So, C-2 carbon is a chirality center.
Want to see more full solutions like this?
Chapter 18 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
- Draw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forwardOrganic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forward
- Differentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forwardDraw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forward
- Answer the question in the first photoarrow_forwardGgggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

