
Concept explainers
For each N-substituted benzene, predict whether the compound reacts faster than, slower than, or at a similar rate to benzene in electrophilic
a. 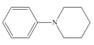 b.
b. 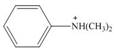 c.
c. 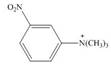 d.
d. 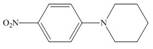
(a)
Interpretation: The given compound reacts faster than, slower than, or at equal rate to benzene in electrophilic aromatic substitution is to be predicted and the major product(s) formed by the reaction between given compound and general electrophile
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.49P
The given compound reacts faster in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
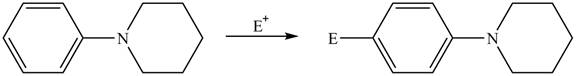
Explanation of Solution
The electron donating groups or activating groups make benzene ring more electron rich, as a result the compound reacts faster in electrophilic substitution reaction than benzene ring. On the other hand, the electron withdrawing groups or deactivating groups make benzene ring less electron rich; as a result the compound reacts slower in electrophilic substitution reaction than benzene ring.
In the given compound, benzene ring is attached to
The activating groups are ortho, para directing whereas the deactivating groups are meta directing. The major product formed by the reaction between given compound and general electrophile
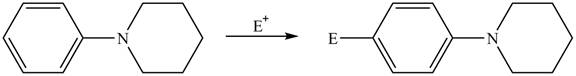
Figure 1
The given compound reacts faster in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
(b)
Interpretation: The given compound reacts faster than, slower than, or at equal rate to benzene in electrophilic aromatic substitution is to be predicted and the major product(s) formed by the reaction between given compound and general electrophile
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.49P
The given compound reacts slower in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
Explanation of Solution
The electron donating groups or activating groups make benzene ring more electron rich, as a result the compound reacts faster in electrophilic substitution reaction than benzene ring. On the other hand, the electron withdrawing groups or deactivating groups make benzene ring less electron rich; as a result the compound reacts slower in electrophilic substitution reaction than benzene ring.
In the given compound, benzene ring is attached to
The activating groups are ortho, para directing whereas the deactivating groups are meta directing. The major product formed by the reaction between given compound and general electrophile

Figure 2
The given compound reacts slower in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
(c)
Interpretation: The given compound reacts faster than, slower than, or at equal rate to benzene in electrophilic aromatic substitution is to be predicted and the major product(s) formed by the reaction between given compound and general electrophile
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.49P
The given compound reacts slower in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
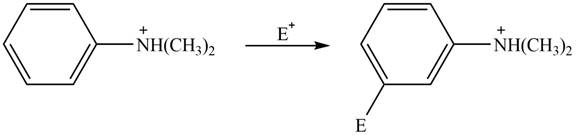
Explanation of Solution
The electron donating groups or activating groups make benzene ring more electron rich, as a result the compound reacts faster in electrophilic substitution reaction than benzene ring. On the other hand, the electron withdrawing groups or deactivating groups make benzene ring less electron rich; as a result the compound reacts slower in electrophilic substitution reaction than benzene ring.
In the given compound, benzene ring is attached to
The activating groups are ortho, para directing whereas the deactivating groups are meta directing. The major product formed by the reaction between given compound and general electrophile
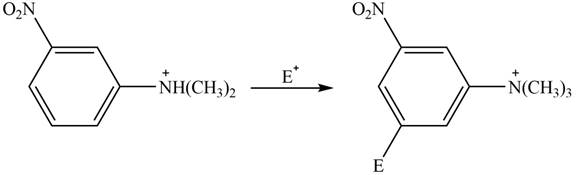
Figure 3
The given compound reacts slower in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
(d)
Interpretation: The given compound reacts faster than, slower than, or at equal rate to benzene in electrophilic aromatic substitution is to be predicted and the major product(s) formed by the reaction between given compound and general electrophile
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.49P
The given compound reacts at a similar rate to benzene in electrophilic substitution reaction. The major product formed by the reaction between given compound and general electrophile
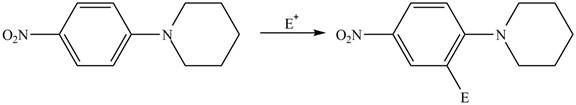
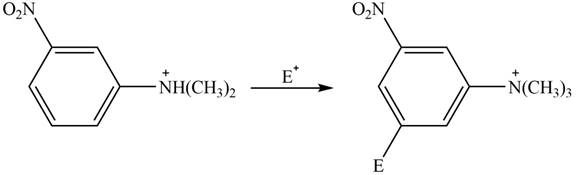
Explanation of Solution
The electron donating groups or activating groups make benzene ring more electron rich, as a result the compound reacts faster in electrophilic substitution reaction than benzene ring. On the other hand, the electron withdrawing groups or deactivating groups make benzene ring less electron rich; as a result the compound reacts slower in electrophilic substitution reaction than benzene ring.
In the given compound, benzene ring is attached to
The activating groups are ortho, para directing whereas the deactivating groups are meta directing. The major product formed by the reaction between given compound and general electrophile

Figure 4
The given compound reacts at a similar rate to benzene in electrophilic substitution reaction. The major product formed by the reaction between given compound and general electrophile
Want to see more full solutions like this?
Chapter 18 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Explain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forward
- Using Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


