
Concept explainers
(a)
Interpretation: The products formed when given compound is treated with
Concept introduction: Benzene undergoes electrophile substitution. The kinetics of the electrophilic substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.16P
The product formed by the reaction of given compound with
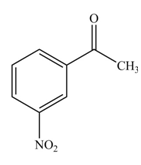
The reaction occurs slower because benzene ring contains deactivating group.
Explanation of Solution
Electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
The substituent present in the given compound is electron withdrawing group. Thus, it directs the electrophile to meta position and deactivates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts slower than benzene. The reaction is shown below.
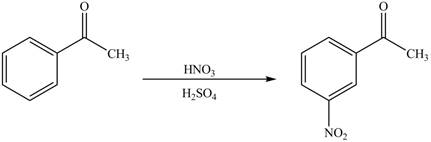
Figure 1
The product formed by the reaction of given compound with
(b)
Interpretation: The products formed when given compound is treated with
Concept introduction: Benzene undergoes electrophile substitution. The kinetics of the electrophilic substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.16P
The product formed by the reaction of given compound with
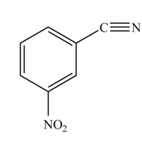
The reaction occurs slower because benzene ring contains deactivating group.
Explanation of Solution
Electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
The substituent present in the given compound is electron withdrawing group. Thus it directs the electrophile to meta position and deactivates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts slower than benzene. The reaction is shown below.
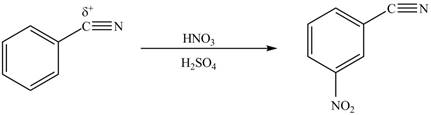
Figure 2
The product formed by the reaction of given compound with
(c)
Interpretation: The products formed when given compound is treated with
Concept introduction: Benzene undergoes electrophile substitution. The kinetics of the electrophile substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.16P
The product formed by the reaction of given compound with
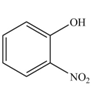
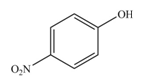
The reaction occurs faster because benzene ring contains activating group.
Explanation of Solution
Electron releasing group directs the electrophile to ortho and para position.
The substituent present in the given compound is electron donating group. Thus, it directs the electrophile to ortho and para position and activates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts faster than benzene. The reaction is shown below.
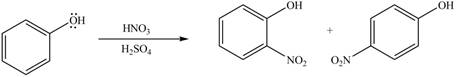
Figure 3
The product formed by the reaction of given compound with
(d)
Interpretation: The products formed when given compound is treated with
Concept introduction: Benzene undergoes electrophile substitution. The kinetics of the electrophilic substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.16P
The products formed by the reaction of given compound with
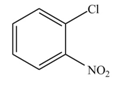
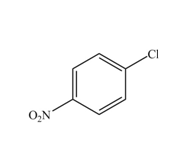
The reaction occurs slower benzene ring because benzene ring contains deactivating group.
Explanation of Solution
The substituent present in the given compound is
Among these two cases, mesomeric effect predominates over inductive effect. Hence, chlorine on benzene ring acts as releasing group but deactivates the benzene ring due to its
Thus, it directs the electrophile to ortho and para position and deactivates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts slower than benzene. The reaction is shown below.
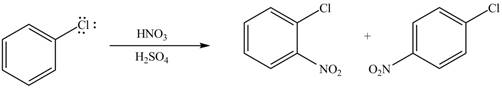
Figure 4
The product formed by the reaction of given compound with
(e)
Interpretation: The products formed when given compound is treated with
Concept introduction: Benzene undergoes electrophile substitution. The kinetics of the electrophile substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.16P
The products formed by the reaction of given compound with
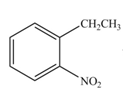
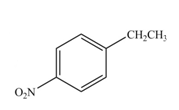
The reaction occurs higher because benzene ring contains activating group.
Explanation of Solution
Electron releasing group directs the electrophile to ortho and para positions.
The substituent present in the given compound is electron withdrawing group. Thus it directs the electrophile to ortho and para positions and activates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts faster than benzene. The reaction is shown below.
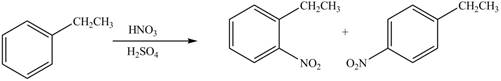
Figure 5
The product formed by the reaction of given compound with
Want to see more full solutions like this?
Chapter 18 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Imagine a four-dimensional world. In it, atoms would have one s orbital and four p orbitals in a given shell. (a) Describe the shape of the Periodic Table of the first 24 elements. (b) What elements would be the first two noble gases (use the names from our world that correspond to the atomic numbers).arrow_forwardThe electron affinity of thulium was measured by a technique called laser photodetachment electron spectroscopy. In this technique, a gaseous beam of anions of an element is bombarded with photons from a laser. The photons knock electrons off some of the anions, and the energies of the emitted electrons are detected. The incident radiation had a wavelength of 1064 nm, and the emitted electrons had an energy of 0.137 eV. Although the analysis is more complicated, we can obtain an estimate of the electron affinity from the energy difference between the photons and the emitted electrons. What is the electron affinity of thulium in electron volts and in kilojoules per mole?arrow_forwardBe sure to answer all parts. The following alkyne is treated with 03 followed by H₂O. Part 1: How many different compounds are formed in this process? 1 Part 2 out of 2 Draw the product of the reaction. draw structure ...arrow_forward
- Many fireworks use magnesium to burn, which releases a significant amount of energy. The heat released causes the oxide to glow, emitting white light. The color of this light can be changed by including nitrates and chlorides of elements that emit in the visible region of their spectra. One such compound is barium nitrate, which produces a yellow-green light. Excited barium ions generate light with wavelengths of 487 nm, 524 nm, 543 nm, and 578 nm. For each case, calculate: (a) the change in energy (in electron volts) of a barium atom and (b) the molar change in energy (in kilojoules per second).arrow_forwardClouds of hot, luminous interstellar hydrogen gas can be seen in some parts of the galaxy. In some hydrogen atoms, electrons are excited to quantum levels with n = 100 or higher. (a) Calculate the wavelength observed on Earth if the electrons fall from the level with n = 100 to one with n = 2. (b) In what series would this transition be found? (c) Some of these high-energy electrons fall into intermediate states, such as n = 90. Would the wavelengths of a transition from the state with n = 100 to one with n = 90 be longer or shorter than those in the Balmer series? Explain your answer.arrow_forwardIn the spectroscopic technique known as photoelectron spectroscopy (PES), ultraviolet radiation is directed at an atom or molecule. Electrons are ejected from the valence shell and their kinetic energies are measured. Since the energy of the incident ultraviolet photons is known and the kinetic energy of the ejected electron is measured, the ionization energy, I, can be deduced because total energy is conserved. (a) Show that the velocity, v, of the ejected electron and the frequency, n, of the incident radiation are related by hv = I + (1/2)mv^2? (b) Use this relation to calculate the ionization energy of a rubidium atom, knowing that light of wavelength 58.4 nm produces electrons with a velocity of 2,450 km/s Recall that 1 J = 1 kg.m^2/s^2arrow_forward
- I) In Millikan's experiment, each droplet observed by the technicians contained an even number of electrons. If they had been unaware of this limitation, how would it have affected their report of an electron's charge?II) Millikan measured the charge of an electron in electrostatic units, esu. The data he collected included the following series of charges found on oil drops: 9.60 X 10^-10 esu, 1.92 X 10^-9 esu; 2.40 X 10^-9 esu; 2.88 X 10^-9 esu; and 4.80 X 10^-9 esu. (a) From this series, find the probable charge of the electron in electrostatic units. (b) Estimate the number of electrons in an oil drop with a charge of 6.72 X 10^-9 esu. The actual charge (in Coulombs) of an electron is 1.602 X 10^-19 C. What is the relationship between esu and Coulombs?arrow_forwardmy ccc edu - Search X Quick Access X D2L Homepage - Spring 2025 x N Netflix X Dimensional Analysis - A x+ pp.aktiv.com Q ☆ X Question 59 of 70 The volume of 1 unit of plasma is 200.0 mL If the recommended dosage for adult patients is 10.0 mL per kg of body mass, how many units are needed for a patient with a body mass of 80.0 kg ? 80.0 kg 10.0 DAL 1 units X X 4.00 units 1 1 Jeg 200.0 DAL L 1 units X 200.0 mL = 4.00 units ADD FACTOR *( ) DELETE ANSWER RESET D 200.0 2.00 1.60 × 10³ 80.0 4.00 0.0400 0.250 10.0 8.00 & mL mL/kg kg units/mL L unit Q Search delete prt sc 111 110 19arrow_forwardIdentify the starting material in the following reaction. Click the "draw structure" button to launch the drawing utility. draw structure ... [1] 0 3 C10H18 [2] CH3SCH3 Harrow_forward
- In an equilibrium mixture of the formation of ammonia from nitrogen and hydrogen, it is found that PNH3 = 0.147 atm, PN2 = 1.41 atm and Pн2 = 6.00 atm. Evaluate Kp and Kc at 500 °C. 2 NH3 (g) N2 (g) + 3 H₂ (g) K₂ = (PN2)(PH2)³ = (1.41) (6.00)³ = 1.41 x 104arrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. and two equivalents of CH2=O draw structure ...arrow_forwardH-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
